Repository for code and simulations from
"Comparison of whole-brain task-modulated functional connectivity methods for fMRI task connectomics"
by Masharipov, R., Knyazeva, I., Korotkov, A., Cherednichenko, D. & Kireev, M.
Use the repository Discussions for questions or email masharipov@ihb.spb.ru
Here, we provide:
-
Task design files (.*mat format) containing stimulus onsets, durations, condition names, and weighting factors for synaptic matrices.
Onsets, durations and condition names are defined in the same way as for multiple conditions *.mat file for SPM12. -
Python code for TMFC simulations based on large-scale Wilson-Cowan neural mass model and Ballon-Windkessel haemodynamic model.
-
User-friendly Jupyter notebooks for reproducing our simulations. Input: task design. Output: simulated BOLD time series.
-
Simulated BOLD time series files (*.mat format) for all experiments presented in the paper.
-
SPM12-based MATLAB code for TMFC analysis using:
- correlation difference approach (CorrDiff),
- standard psychophysiological interactions (sPPI),
- generalised psychophysiological interactions (gPPI),
- correlational psychophysiological interactions (cPPI),
- beta-series correlations based on least-squares-all approach (BSC-LSA),
- beta-series correlations based on least-squares-separate approach (BSC-LSS).
Our simulation approach was based on the coupled oscillator model for functional connectivity (see Mateo et al., 2017), according to which functional connectivity measured by fMRI arises from a correlation between ultra-slow BOLD oscillations caused by ultra-slow modulation of the envelopes of synchronised gamma-band oscillations:
Gamma-band oscillations are linked to sensory processing1,2, motor acts3, and cognitive processes4,5 and are thought to underlie information processing and transmission6,7. The spectral power (or envelope) of gamma-band oscillations fluctuates very slowly with time, and brain regions with shared function demonstrate co-fluctuation of gamma-band envelopes8,9. Animal and human studies have shown that local field potential power in the gamma band is the closest electrophysiological correlate of spontaneous and evoked BOLD signals9,10,11,12,13.
Mateo et al. (2017) appplied optogenetic manipulations and concurrently measured local field potential, arteriole diameter and blood oxygenation in awake mice. They provided direct evidence that an increase in gamma-band power leads to an increase in arteriole diameter, and an increase in arteriole diameter leads to an increase in blood oxygenation. In accordance with previous empirical observations, we observed strong correlations between simulated ultra-slow fluctuations of the gamma-band envelope and time-shifted BOLD signal:
The simulation procedure included five steps:
-
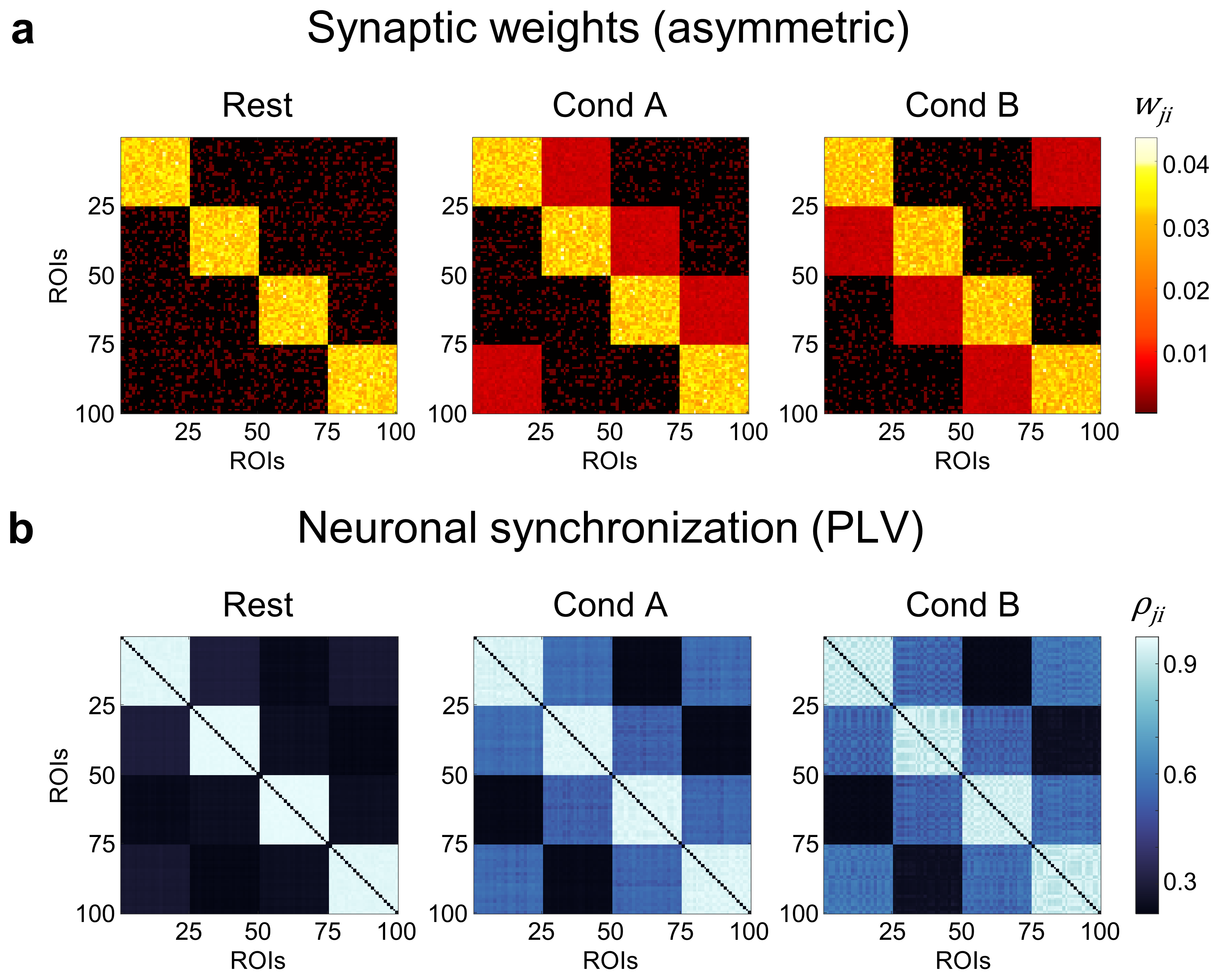
Simulation of gamma-band oscillatory neuronal activity for 100 interconnected brain regions using Wilson-Cowan equations. Synaptic weights between 100 brain regions depend on the task conditions, which allow to control the ground-truth TMFC. Transient activity-dependent modulation of synaptic strength, lasting from tens of milliseconds to several minutes, is referred to as short-term synaptic plasticity14,15,16.
-
Simulation of simple co-activations using box-car functions. Co-activations are simultaneous activations (task-evoked hemodynamic responses) without communication between brain regions.
-
Using the Balloon-Windkessel haemodynamic model to convert oscillatory neuronal activity and co-activations into BOLD signals.
-
Downsampling of the BOLD signal to different time resolutions.
-
Adding white Gaussian noise to model scanner measurement error. This step was done in MATLAB (see MATLAB_code folder).
BOLD signal related to co-activations was normalised such that the ratio between the standard deviations of the oscillatory-related signal and co-activation-related signal was determined by the scaling factor: SF = σoscill/σcoact.
The signal-to-noise ratio (SNR) was defined as the ratio between the standard deviations of the signal and noise: SNR = σsignal/σnoise.
Tasks consisted of two task conditions (A and B) interleaved by rest periods. By default, we considered symmetric synaptic weight matrices:
In the last simulation experiment, we used asymmetric synaptic weight matrices to test whether the gPPI method could be used to obtain at least some information about the direction of connectivity:
We first considered simulations without co-activations (SF = 0) to investigate whether different TMFC methods produce FC matrices similar to ground-truth synaptic weight matrices for a sample size N = 100, SNR = 0.4, and TR = 2 s. pFDR < 0.001.
Correlation difference approach (CorrDiff):
Standard psychophysiological interactions (sPPI) with deconvolution (with and without sPPI matrix symmetrisation):
Standard psychophysiological interactions (sPPI) without deconvolution (with and without sPPI matrix symmetrisation):
Generalised psychophysiological interactions (gPPI) with deconvolution (with and without gPPI matrix symmetrisation):
Generalised psychophysiological interactions (gPPI) without deconvolution (with and without gPPI matrix symmetrisation):
Beta-series correlations based on least-squares-all approach (BSC-LSA):
Beta-series correlations based on least-squares-separate approach (BSC-LSS):
Correlational psychophysiological interactions (cPPI):
Task-state functional connectivity (TSFC) and background functional connectivity (BGFC):
Next, we considered simulations with co-activations (SF = 1) to investigate how different TMFC methods address artificial inflation of TMFC estimates due to simultaneous activation of brain regions without task-related modulation of synaptic weights between them:
gPPI without deconvolution (e.g. gPPI without deconvolution is implemented in the FSL and CONN toolbox):
Here we can see that gPPI without deconvolution does not protect against co-activations.
To isolate TMFC from co-activation effects, we can regress out task activations using finite impulse response (FIR) functions prior to TMFC analysis. FIR task regression substantially improves gPPI specificity:
Finally, we used asymmetric synaptic weight matrices to test whether the gPPI method has at least some causal interpretability. Asymmetric synaptic weight matrices should be reflected by task-modulated effective connectivity (TMEC):
The regression dynamic causal modelling (rDCM) method (see Note), which is a conventional EC method, was used as a reference. As rDCM requires a relatively high SNR, we used SNR = 5 here. If the gPPI method is unable to correctly evaluate the direction of information flow at a high SNR, then it would also fail at lower SNRs.
Results for block design with twenty 20 s blocks per condition and SNR = 5:
Here we can see that rDCM and gPPI with deconvolution are able to determine the effective strength of task-modulated synaptic connections at high SNR and long scan durations.
Note: Original DCM method enables us to estimate the effective strength of task-independent (intrinsic) synaptic connections (A matrix), task-modulated (extrinsic) synaptic connections (B matrix), and the direct influence of driving inputs that cause activations (C matrix). The task-modulated effective connectivity (TMEC) matrix cannot be directly obtained using the rDCM approach because it is based on the linear neural state equation without the B matrix. If we feed the entire time series of the resting-state or task-state BOLD signal into rDCM, the A matrix will reflect the resting-state effective connectivity (RSEC) and task-state effective connectivity (TSEC) matrices, respectively. In the latter case, the A matrix will depend on both spontaneous (intrinsic) and task-modulated (extrinsic) oscillations. To calculate the TMEC matrix, we propose calculating two A matrices for concatenated “Cond A” and “Cond B” block time series after removing the first six seconds of each block. The difference between these matrices will subtract spontaneous (intrinsic) EC and result in TMEC: