This repository contains the code for the following paper:
Lovro Vrček, Xavier Bresson, Thomas Laurent, Martin Schmitz, Mile Šikić. Learning to Untangle Genome Assembly with Graph Convolutional Networks, arXiv preprint arXiv:2206.00668, 2022.
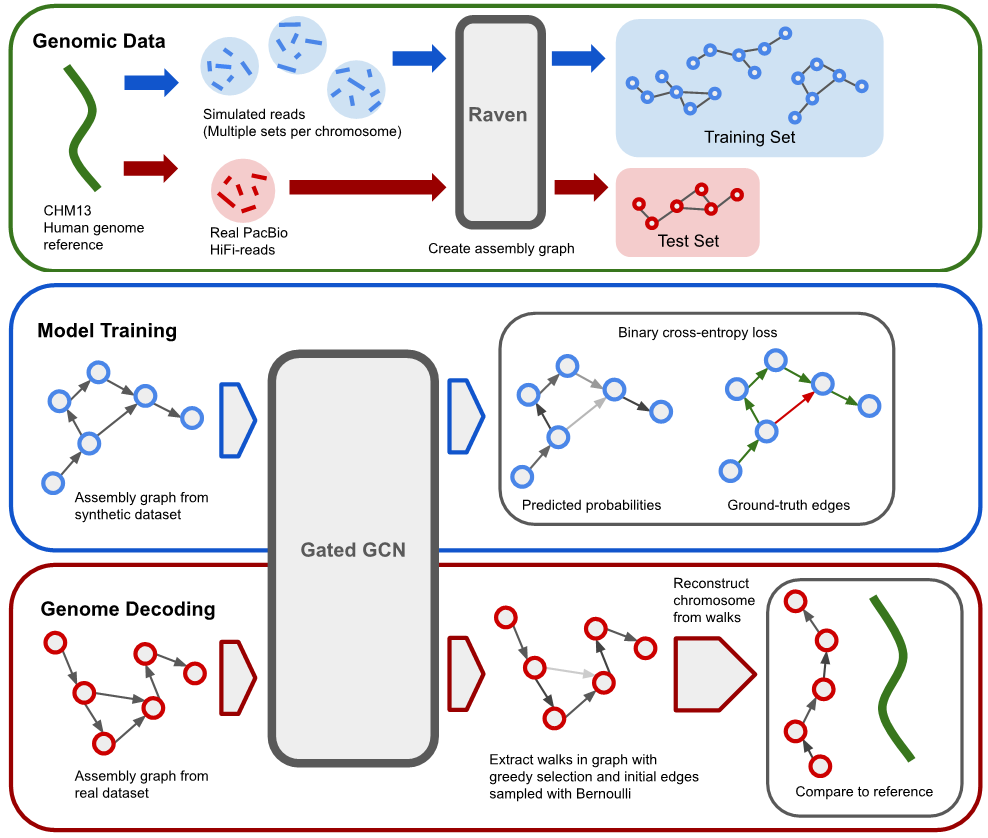
A framework for training graph neural networks to untangle assembly graphs obtained from de novo genome assembly.
This framework consists of simulating the synthetic reads, generating the assebmly graphs, training a Graph Neural Network to predict edge probabilities, and finally decoding these edge probabilities with greedy search. The final result are assembly sequences stored in the commonly used FASTA format.
- conda: 4.6+
conda env create -f environment.yml
conda activate gnn-assemblyUse pip for both of the installations bellow.
-
PyTorch: Install version 1.9 or higher, based on your CUDA version: https://pytorch.org/get-started/previous-versions/#linux-and-windows-7
-
DGL: Install version 3.8 or higher, based on your CUDA version: https://www.dgl.ai/pages/start.html
For example, for CUDA 11.1, this could be:
pip install torch==1.9.0+cu111 torchvision==0.10.0+cu111 torchaudio==0.9.0 -f https://download.pytorch.org/whl/torch_stable.html
pip install dgl-cu111 dglgo -f https://data.dgl.ai/wheels/repo.htmlTo run a quick example, run:
python example.pyThis will also download the CHM13 reference and set up the directory structure for simulating reads, constructing graphs and running experiments. Default location is in the data directory of the root directory of this project.
Apart from setting up the working directory, running the above example will simulate four read datasets for chromosome 19 and one read dataset for chromosome 21 and construct graphs from those reads. Subsequently, it will train a model on three chromosome 19 graphs, validate it on one chromosome 19 graph, and, finally, create assembly for chromosome 21. After reconstruction, the assembly sequences can be found in data/experiments/test_example/assembly/0_assembly.fasta.
The real data is automatically downloaded by running the example.py script, as described above or if you run a custom run with pipeline.py, as described in the next section. However, you can also download the real dataset separately, by running:
bash download_dataset.sh <data_path>after which the dataset will be saved in <data_path>/real/
Note: This dataset has 43 GB when compressed (during download) and 180 GB when uncompressed (used during training/evaluation).
The results can easily be reproduced by running reproduce.py script, with --mode argument set to either synth for synthetic data or real for real data. For example:
python reproduce.py --mode realwill load the model pretrained on 15 chromosome 19 graphs, which is stored in pretrained_models/model_15xchr19.pt, and produce assembly sequences for all 23 real-data chromosomes. We suggest you evaluate the obtained assemblies with Quast, as explained at the end of this README.
You can choose on which graphs to train, validate, and test, by editing _train_dict, valid_dict, and _test_dict inside config.py.
Inside each dictionary specify how many graphs created from which chromosome you wish to train, validate, and test on. For real data, add suffix "_r".
E.g., to train on two graphs of chromosome 19 and one graphs of chromosome 20, valdiate on one chromosome 19 graphs, and test on chromosome 21 graph created from real PacBio HiFi data, write:
_train_dict = {'chr19': 2, 'chr20': 1}
_valid_dict = {'chr19': 1}
_test_dict = {'chr21_r': 1}Note that all three chr19 graphs are created from different sets of reads (sampled anew each time), and thus are different themselves. Also note that you cannot specify more than one graph per real chromosome.
All the hyperparameters are in the hyperparameters.py. Change them by editing the dictionary inside the file.
python pipeline.py --data <data_path> --out <out_name>Arguments:
-
--data: (default value:data/) path where simulated sequences, real sequences, constructed graphs, and the experiments will be stores. -
--out: (default value: timestamp at running the pipeline) name that will be used for naming the directories for train/valid/test, and saving the model and the checkpoints during the training. The models are saved inside thepretraineddirectory. E.g., with--out example, the model will be saved intpretrained/model_example.pt, and all the checkpoint information (optimizer parameters, loss, etc.) will be saved insidecheckpoints/example.pt. In the same example, train/valid/test directories would be<data_path>/experiments/train_example,<data_path>/experiments/valid_example,<data_path>/experiments/test_example.
For example, if you want to save the data inside other_dir/data and call all the output files example_run, run:
python pipeline.py --data other_dir/data --out example_runThe assembly sequences, obtained for the graphs in the test set, wil lbe stored inside <data_path>/experiments/test_<out>/assembly directory. The easiest way to evaluate the obtained assemblies is with Quast. For installing Quast, we suggest creating a new conda environment (due to clashing dependencies):
conda create -n quast python=3.6
conda activate quast
conda install -c bioconda quastThe general usage is as follows:
quast -r <path_to_reference> -o <output_dir> <path_to_assembly_file>For example, if inside test_example directory you only have one graph of chromosome 21, then you could evaluate the assembly sequence of that chromosome by running:
quast -r <ref_path>/chromosomes/chr21.fasta -o <data_path>/experiments/test_example/quast <data_path>/experiments/test_example/assembly/0_assembly.fastaThe report, containing all the evaluation metrics, will be located at <data_path>/experiments/test_example/quast/report.txt.
To cite this work, please use the following:
@article{vrvcek2022learning,
title={Learning to Untangle Genome Assembly with Graph Convolutional Networks},
author={Vr{\v{c}}ek, Lovro and Bresson, Xavier and Laurent, Thomas and Schmitz, Martin and {\v{S}}iki{\'c}, Mile},
journal={arXiv preprint arXiv:2206.00668},
year={2022}
}