Welcome to xportr! We have designed xportr to help get your xpt
files ready for transport either to a clinical data set validator
application or to a regulatory agency This package has the functionality
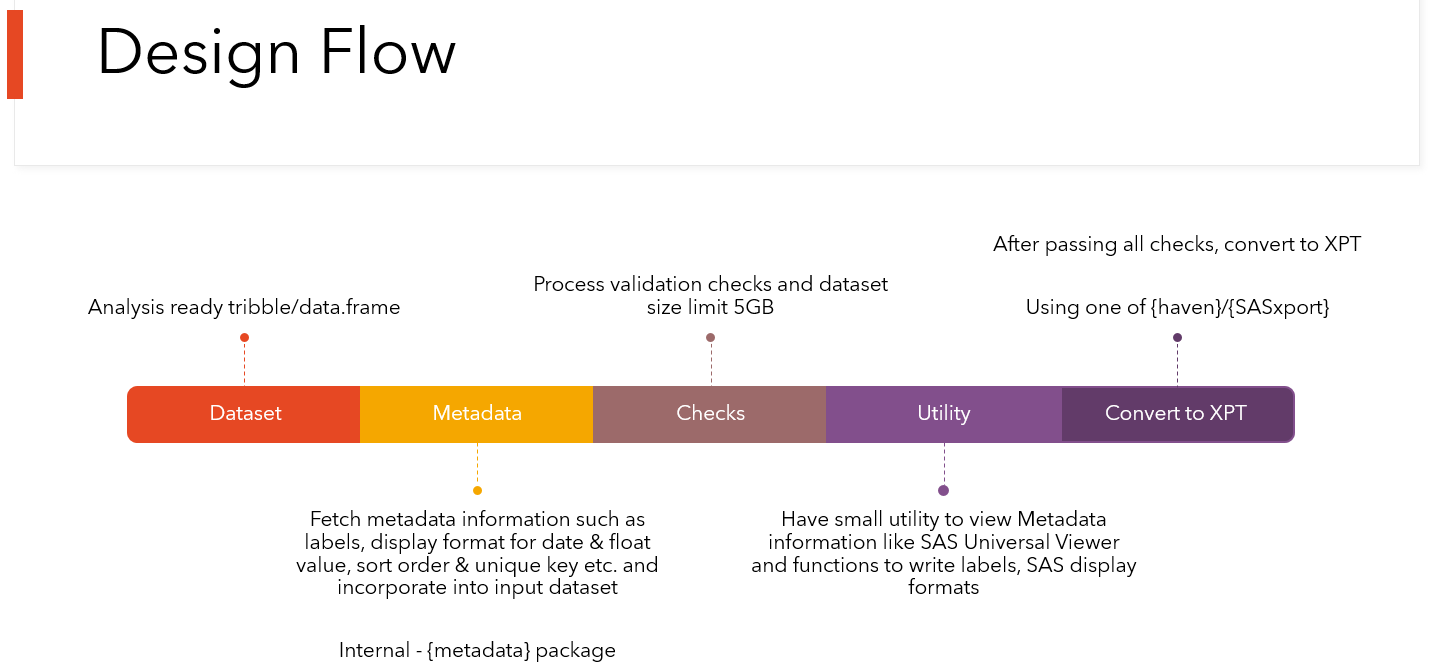
to associate all metadata information to a local R data frame, perform
data set level validation checks and convert into a transport v5
file(xpt).
As always, we welcome your feedback. If you spot a bug, would like to see a new feature, or if any documentation is unclear - submit an issue on xportr’s Github page.
devtools::install_github("https://github.com/atorus-research/xportr.git")- As this is an experimental package and under development we have not made it available on CRAN.
xportr is designed for clinical programmers to create CDISC compliant
xpt files- ADaM or SDTM. Essentially, this package has two big
components to it - writing xpt files with well-defined metadata and
checking compliance of the data sets. The first set of tools are
designed to allow a clinical programmer to build a CDISC compliant xpt
file directly from R. The second set of tools are to perform checks on
your data sets before you send them off to any validators or data
reviewers.
- Variable names must start with a letter.
- Variables names are ≤ 8 characters.
- Allotted length for each column containing character (text) data should be set to the maximum length of the variable used across all data sets (≤ 200)
- Coerces variables to only numeric or character types
- Display format support for numeric float and date/time values
- Variable labels are ≤ 200 characters.
- Data set labels are ≤ 40 characters.
- Presence of non-ASCII characters in Variable Names, Labels or data set labels.
NOTE: Each check has associated messages and warning.
The first example involves an ADSL data set in the .sas7bdat format
with associated specification in the .xlsx format.
adsl <- haven::read_sas("inst/extdata/adsl.sas7bdat")
var_spec <- readxl::read_xlsx("inst/specs/ADaM_spec.xlsx", sheet = "Variables") %>%
dplyr::rename(type = "Data Type") %>%
rlang::set_names(tolower)
data_spec <- readxl::read_xlsx("inst/specs/ADaM_spec.xlsx", sheet = "Datasets") %>%
rlang::set_names(tolower) %>%
dplyr::rename(label = "description")
adsl %>%
xportr_type(var_spec, "ADSL", "message") %>%
xportr_length(var_spec, "ADSL", "message") %>%
xportr_label(var_spec, "ADSL", "message") %>%
xportr_df_label(data_spec, "ADSL") %>%
xportr_write("adsl.xpt")Please check out the Get Started for more information.
We are in talks with other Pharma companies involved with the
{pharmaverse} to enhance this package to play well with other
downstream and upstream packages.