1. Setup CCN
2. Broad Training CCN
3. Broad Validation CCN
4. Subclass Training CCN
5. Subclass Validation CCN
6. Application of CCN
7. Other Tools
8. Old way of Training
9. GRN Construction
10. GRN Status
11. TF Scores
12. scRNA-seq Aggregation
CancerCellNet is a R package that allows cancer type classification and evaluation of transcriptional fidelity for cancer models across species and platform (bulk RNA-seq, microarray). Alternatively, you can visit our web-app. You can also read about the applications of CancerCellNet in our publication.
We will demonstrate how to
- build/apply general (broad) classifier
- build/apply subclass classifier
- reconstruct gene regulatory network
- calcuate GRN status
- calculate TF scores
library(devtools)
install_github("pcahan1/cancerCellNet", ref="master", auth="your_token_here")
install.packages("pheatmap")
install.packages("RColorBrewer")
install.packages("randomForest")
install.packages("ggplot2")
install.packages("igraph")
install.packages("stringr")
install.packages("snow")
# install packages from Bioconductor
if (!requireNamespace("BiocManager", quietly = TRUE))
install.packages("BiocManager")
BiocManager::install("AnnotationDbi")
BiocManager::install("GO.db")
BiocManager::install("org.Hs.eg.db")All the training data were compiled from the TCGA project. The example expression profiles of UCEC cell lines were extracted from CCLE. The example expression profiles of UCEC GEMMs were taken from the study Blaisdell et al, 2015 (GSE73541)
# fetch compiled TCGA training data
download.file("https://cnobjects.s3.amazonaws.com/cancerCellNet/resources/expGDC_compiled.rda", "Named_expGDC_20181218.rda")
download.file("https://cnobjects.s3.amazonaws.com/cancerCellNet/resources/stGDC_compiled.rda", "Named_stGDC_20181218.rda")
# fetch sample cancer models
download.file("https://cnobjects.s3.amazonaws.com/cancerCellNet/resources/CCLE_UCEC.rda", "CCLE_UCEC.rda")
download.file("https://cnobjects.s3.amazonaws.com/cancerCellNet/resources/GEMM_UCEC.rda", "GEMM_UCEC.rda")
# fetch data needed for subclass training
download.file("https://cnobjects.s3.amazonaws.com/cancerCellNet/resources/UCEC_readyToTrain_sub_exp.rda", "UCEC_readyToTrain_sub_exp.rda")
download.file("https://cnobjects.s3.amazonaws.com/cancerCellNet/resources/UCEC_readyToTrain_sub_st.rda", "UCEC_readyToTrain_sub_st.rda")
download.file("https://cnobjects.s3.amazonaws.com/cancerCellNet/resources/iGenes.rda", "iGenes.rda")
Load in the necessary files first.
library(cancerCellNet)
expGDC = utils_loadObject("expGDC_compiled.rda")
stGDC = utils_loadObject("stGDC_compiled.rda")
CCLE_sample = utils_loadObject("CCLE_UCEC.rda")
GEMM_sample = utils_loadObject("GEMM_UCEC.rda")
iGenes = Reduce(intersect, list(rownames(CCLE_sample), rownames(GEMM_sample), rownames(expGDC)))
save(iGenes, file = "iGenes.rda")
stList = splitCommon_proportion(sampTab = stGDC, proportion = 0.66, dLevel = "project_id")
stTrain = stList$trainingSet
expTrain = expGDC[iGenes, stTrain$barcode]
Because the training data is not balanced in this case, we would have to use stratified sampling in this case. The samplesize parameter indicates the samplesize of stratified sampling. Additionally, because the process of gene pair transform is resource intensive and time consuming, we developed a modified method to perform quick pair transform that is much quicker.
broad_return = broadClass_train(stTrain = stTrain,
expTrain = expTrain,
colName_cat = "project_id",
colName_samp = "barcode",
nRand = 70,
nTopGenes = 30,
nTopGenePairs = 75,
nTrees = 2000,
stratify=TRUE,
sampsize=60,
quickPairs=TRUE)
stVal_Broad = stList$validationSet
stVal_Broad_ord = stVal_Broad[order(stVal_Broad$project_id), ] #order by broadClass
stVal_Broad[order(stVal_Broad$project_id), ] #order by broadClass
expVal_Broad = expGDC[iGenes, rownames(stVal_Broad_ord)]
cnProc_broad = broad_return$cnProc #select the cnProc from the broadclass training earlier
classMatrix_broad = broadClass_predict(cnProc_broad, expVal_Broad, nrand = 60)
stValRand_broad = addRandToSampTab(classMatrix_broad, stVal_Broad_ord, "project_id", "barcode")
grps = as.vector(stValRand_broad$project_id)
names(grps)<-rownames(stValRand_broad)
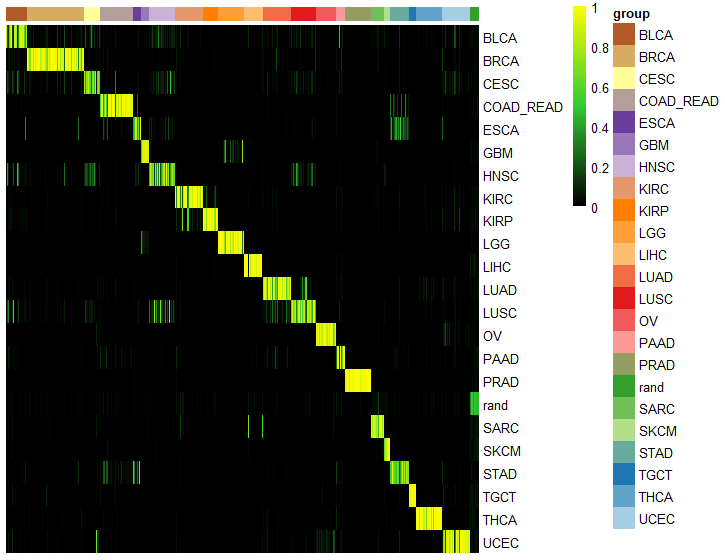
ccn_hmClass(classMatrix_broad, grps=grps, fontsize_row=10)
breakVector = c() # create a vector of number indicating the column at which the gap will be placed
for (uniqueClass in unique(grps)) {
myBreak = max(which(grps %in% uniqueClass))
breakVector = c(breakVector, myBreak)
}
ccn_hmClass(classMatrix_broad, grps=grps, fontsize_row=10, gaps_col = breakVector)
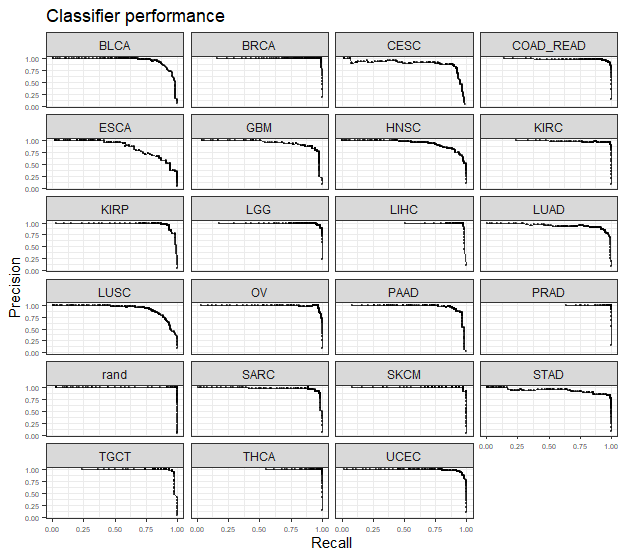
assessmentDat = ccn_classAssess(classMatrix_broad, stValRand_broad, "project_id","barcode")
plot_class_PRs(assessmentDat)
Now that we see the broad class classifier has good performance, we can train a broad classifier with all the data.
broad_return = broadClass_train(stTrain = stGDC,
expTrain = expGDC[iGenes, ],
colName_cat = "project_id",
colName_samp = "barcode",
nRand = 70,
nTopGenes = 30,
nTopGenePairs = 75,
nTrees = 2000,
stratify=TRUE,
sampsize=60,
quickPairs=TRUE)
save(broad_return, file="BroadClassifier_return.rda")
expGDC_sub = utils_loadObject("UCEC_readyToTrain_sub_exp.rda")
stGDC_sub = utils_loadObject("UCEC_readyToTrain_sub_st.rda")
returnBroad = utils_loadObject("BroadClassifier_return.rda")
iGenes = utils_loadObject("iGenes.rda")
stList_sub = splitCommon_proportion(sampTab = stGDC_sub, proportion = 0.66, dLevel = "subClass")
stTrain_sub = stList_sub$trainingSet
expTrain_sub = expGDC_sub[iGenes, as.vector(stTrain_sub$samples)]
cnProc_broad = returnBroad$cnProc
In this case, majority of the rand profiles are generated from other TCGA cancer samples. But, you can still add some truly permutated profiles into the training. You can also adjust the weight of broad class classification scores as features.
returnSubClass = subClass_train(cnProc_broad = cnProc_broad, stratify = TRUE, sampsize = 15,
stTrain = stTrain_sub,
expTrain = expTrain_sub,
colName_broadCat = "broadClass",
colName_subClass = "subClass",
name_broadCat = "TCGA-UCEC",
weight_broadClass = 5,
colName_samp="samples",
nRand = 15,
nTopGenes = 30,
nTopGenePairs = 50,
nTrees = 1000)
stVal_Sub = stList_sub$validationSet
# to get a more even validation...better for visualizing
stVal_split = splitCommon(sampTab = stVal_Sub, ncells = 8, dLevel = "subClass")
stVal_Sub = stVal_split$train #even though it says train, it merely contain an equally sampled dataset
stVal_Sub_ord = stVal_Sub[order(stVal_Sub$subClass), ] #order by cateogry
expVal_sub = expGDC_sub[iGenes, rownames(stVal_Sub_ord)]
cnProc_sub = returnSubClass$cnProc
classMatrix_sub = subClass_predict(cnProc_broad, cnProc_sub, expVal_sub, nrand = 2, weight_broadClass = 10)
stValRand_sub = addRandToSampTab(classMatrix_sub, stVal_Sub_ord, "subClass", "samples")
grps = as.vector(stValRand_sub$subClass)
names(grps) = rownames(stValRand_sub)
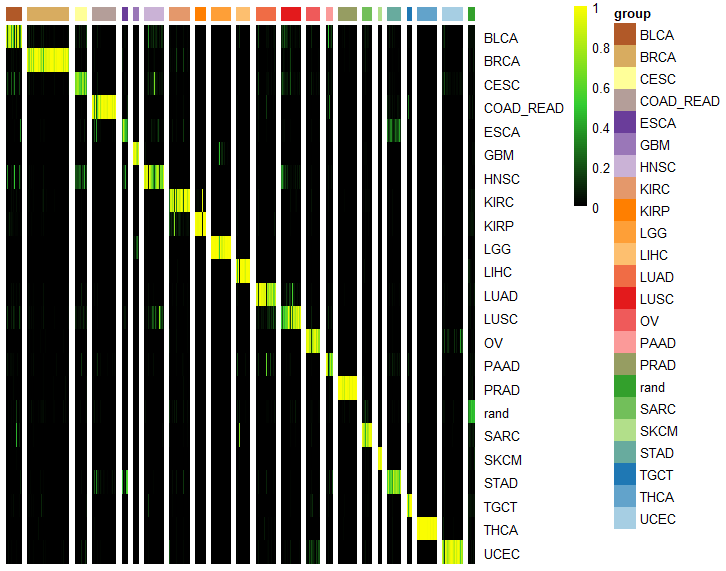
ccn_hmClass(classMatrix_sub, grps=grps, fontsize_row=10)
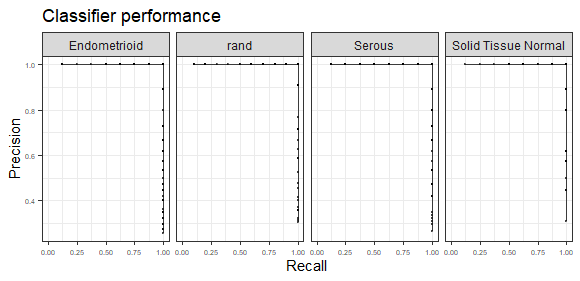
assessmentDat = ccn_classAssess(classMatrix_sub, stValRand_sub, "subClass","samples")
plot_class_PRs(assessmentDat) # plot out the PR curves
returnSubClass = subClass_train(cnProc_broad = cnProc_broad, stratify = TRUE, sampsize = 15,
stTrain = stTrain_sub,
expTrain = expTrain_sub,
colName_broadCat = "broadClass",
colName_subClass = "subClass",
name_broadCat = "TCGA-UCEC",
weight_broadClass = 5,
colName_samp="samples",
nRand = 15,
nTopGenes = 30,
nTopGenePairs = 50,
nTrees = 1000)
save(returnSubClass, file = "subClass_UCEC_return.rda")
CCLE_sample = utils_loadObject("CCLE_UCEC.rda")
GEMM_sample = utils_loadObject("GEMM_UCEC.rda")
returnBroad = utils_loadObject("BroadClassifier_return.rda")
returnSubClass = utils_loadObject("subClass_UCEC_return.rda")
cnProc_broad = returnBroad$cnProc
cnProc_subclass = returnSubClass$cnProc_subClass
classMatrix_CCLE = broadClass_predict(cnProc = cnProc_broad, expDat = CCLE_sample, nrand = 2)
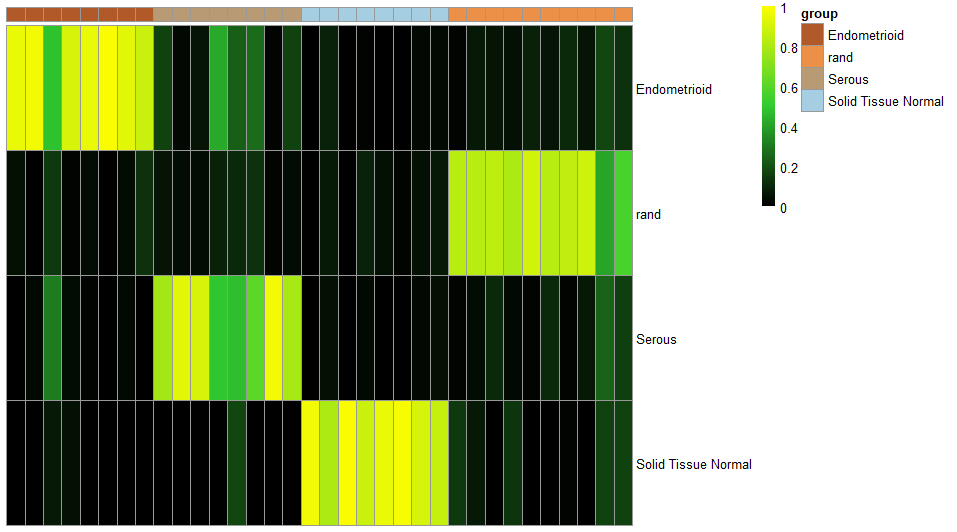
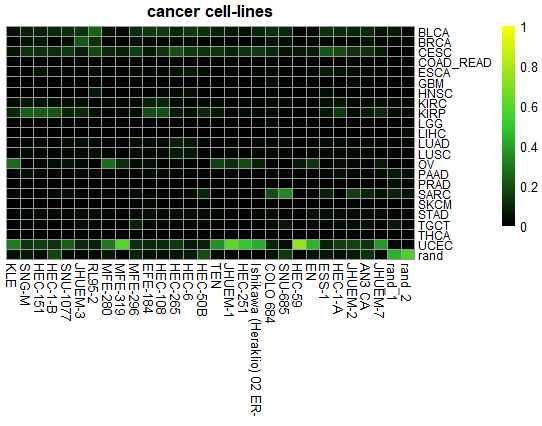
ccn_hmClass(classMatrix_CCLE, main = "cancer cell-lines", fontsize_row=9, fontsize_col = 10)
classMatrix_CCLE_sub = subClass_predict(cnProc = cnProc_broad, cnProc_sub = cnProc_subclass, weight_broadClass = 5, expDat = CCLE_sample, nrand = 2)
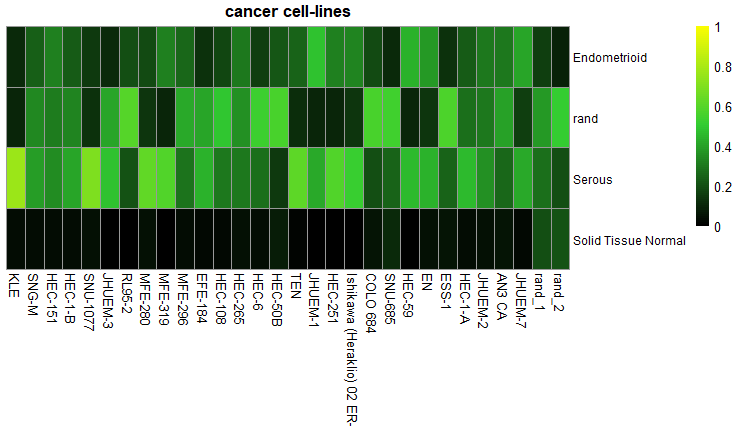
ccn_hmClass(classMatrix_CCLE_sub, main = "cancer cell-lines", fontsize_row=9, fontsize_col = 10)
You can also apply it to GEMM samples provided above. For classifiying other GEMM samples, you may have to find the human orthologous genes between mouse and human. We built a function that can do the conversion listed below. But you can also use biomaRt to perform conversion.
postConversionExpMatrix = utils_convertToGeneSymbols(expTab = preConversionExpressionMatrix, typeMusGene = TRUE)
CCLE_sample = utils_loadObject("CCLE_UCEC.rda")
returnSubClass = utils_loadObject("subClass_UCEC_return.rda")
expGDC_sub = utils_loadObject("UCEC_readyToTrain_sub_exp.rda")
stGDC_sub = utils_loadObject("UCEC_readyToTrain_sub_st.rda")
genePairs = returnSubClass$cnProc_subClass$xpairs
# generate gene pairs
expTransform = query_transform(expGDC_sub, genePairs)
# average the genepair signals among TCGA samples in a category
avgGenePair_TCGA = avgGeneCat(expDat = expTransform, sampTab = stGDC_sub, dLevel = "subClass", sampID = "samples")
genePairs_query = query_transform(CCLE_sample, genePairs)
geneCompareMatrix = makeGeneCompareTab(queryExpTab = genePairs_query,
avgGeneTab = avgGenePair_TCGA, geneSamples = genePairs)
plotGeneComparison(geneCompareMatrix, fontsize_row = 7)
CCLE_sample = utils_loadObject("CCLE_UCEC.rda")
returnSubClass = utils_loadObject("subClass_UCEC_return.rda")
expGDC_sub = utils_loadObject("UCEC_readyToTrain_sub_exp.rda")
stGDC_sub = utils_loadObject("UCEC_readyToTrain_sub_st.rda")
iGenes = utils_loadObject("iGenes.rda")
cGenesList = returnSubClass$cgenes_list
# get the genes that contribute to the creating of genepairs
genePairs = returnSubClass$cnProc_subClass$xpairs
cgenes = strsplit(x = genePairs, split = "_")
cgenes = unique(unlist(cgenes))
# create annotation table of classy genes and its corresponding category
annoDf = data.frame(matrix(nrow = length(cgenes), ncol = 1))
colnames(annoDf) = "ClassificationCat"
rownames(annoDf) = cgenes
for (setName in names(cGenesList)) {
geneSet = cGenesList[[setName]]
annoDf[rownames(annoDf) %in% geneSet, "ClassificationCat"] = setName
}
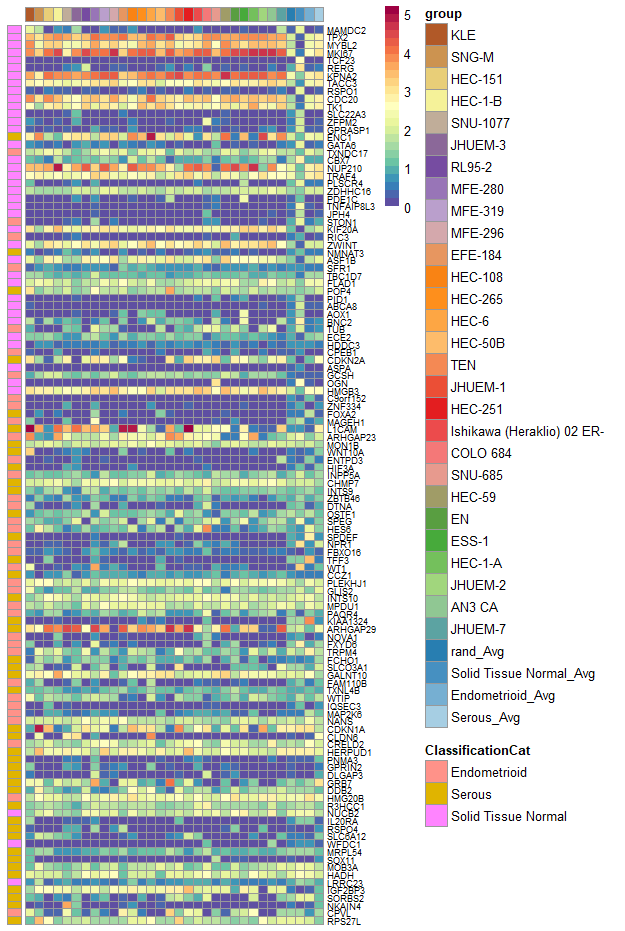
expNorm = trans_prop(weighted_down(expGDC_sub[iGenes, ], 5e5, dThresh=0.25), 1e5)
# average the gene expression among TCGA samples in a category
avgGene_TCGA = avgGeneCat(expDat = expNorm, sampTab = stGDC_sub, dLevel = "subClass", sampID = "samples")
query_expNorm = trans_prop(weighted_down(CCLE_sample[iGenes, ], 5e5, dThresh=0.25), 1e5)
geneCompareMatrix = makeGeneCompareTab(queryExpTab = query_expNorm,
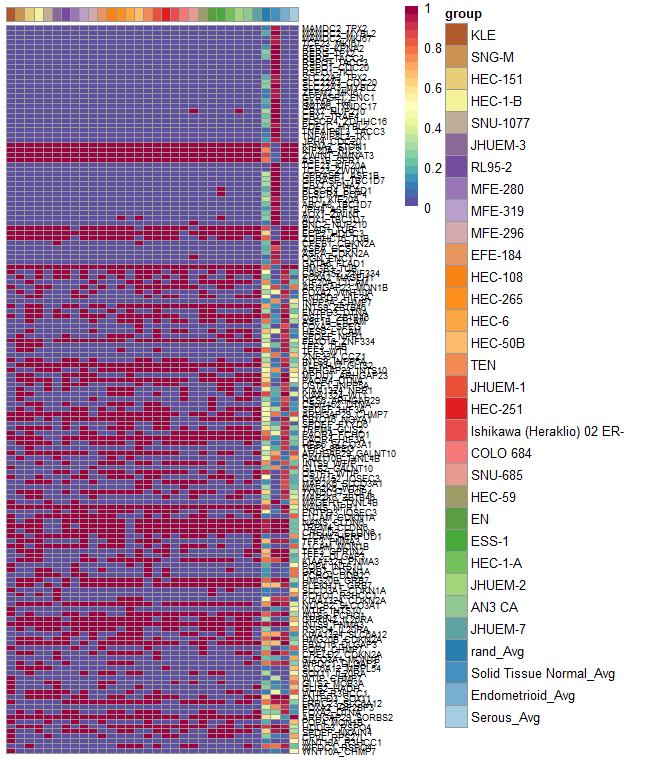
avgGeneTab = avgGene_TCGA, geneSamples = cgenes)
plotGeneComparison(geneCompareMatrix[rownames(annoDf), ], fontsize_row = 7, annotation_row = annoDf)
The old way of training instead of having one packaged function
library(cancerCellNet)
expGDC = utils_loadObject("Named_expGDC_20181218.rda")
stGDC = utils_loadObject("Named_stGDC_20181218.rda")
CCLE_sample = utils_loadObject("CCLE_UCEC.rda")
GEMM_sample = utils_loadObject("GEMM_UCEC.rda")
iGenes = Reduce(intersect, list(rownames(CCLE_sample), rownames(GEMM_sample), rownames(expGDC)))
expGDC = expGDC[iGenes, ]
The old way is basically running functions packed in broadClass_Train function individually.
expTnorm = trans_prop(weighted_down(expTrain, 5e5, dThresh=0.25), 1e5)
# find genes with different expression in each cancer category
system.time(cgenes<-findClassyGenes(expTnorm, stTrain, "description2", topX=20))
cgenesA = cgenes[['cgenes']]
grps = cgenes[['grps']]
cgenes_list = cgenes[['labelled_cgenes']]
# find top differentiating gene pairs
system.time(xpairs<-ptGetTop(expTrain[cgenesA,], grps, cgenes_list, topX=50, sliceSize=2000, quickPairs=TRUE)) # if you don't want quick pairs, turn it off.
# some of these might include selection cassettes; remove them
xi = setdiff(1:length(xpairs), grep("selection", xpairs))
xpairs = xpairs[xi]
# pair transform training data
system.time(pdTrain<-query_transform(expTrain[cgenesA, ], xpairs))
tspRF = makeClassifier(pdTrain[xpairs,], genes=xpairs, groups=grps, nRand = 20, ntrees = 2000, stratify=TRUE, sampsize=60)
cnProc = list("cgenes"= cgenesA, "xpairs"=xpairs, "grps"= grps, "classifier" = tspRF)
# after the cnProc is generated, you can save it and use it to perform classification. This is a slightly less storage method of training
The GRN reconstruction method is based on our previously developed method that can be found here.
library(cancerCellNet)
# load in the training samples and intersecting genes between training sample and query samples
expGDC = utils_loadObject("expGDC_compiled.rda")
stGDC = utils_loadObject("stGDC_compiled.rda")
iGenes = utils_loadObject("iGenes.rda")
expGDC = expGDC[iGenes, ]
# evenly samples 80 samples per cancer type for training
stList = splitCommon(sampTab = stGDC, ncells = 60, dLevel = "project_id")
stTrain = stList$train
save(stList, file = "stList_grn.rda")
expTrain = expGDC[,rownames(stTrain)]
# normalize the training data
expTrain = trans_prop(weighted_down(expTrain, 5e5, dThresh=0.25), 1e5)
rm(list = c("expGDC", "stGDC"))
# GRN reconstruction
grnAll = ccn_makeGRN(expTrain, stTrain, "project_id", zThresh = 4, dLevelGK = NULL, prune = TRUE, holm = 1e-4, cval=0.3)
save(grnAll, file = "grnAll.rda")
Different from our previously developed method, we devised a method that uses the rank of gene epxressions rather than the expression values.
library(cancerCellNet)
library(ggplot2)
# load in training expression file
expGDC = utils_loadObject("expGDC_compiled.rda")
iGenes = utils_loadObject("iGenes.rda")
# load in splitted training sample table
stList = utils_loadObject("stList_grn.rda")
expTrain = expGDC[iGenes, rownames(stList$train)]
stTrain = stList$train
# rank the genes then log the rank. We found that it works pretty well with just ranking the genes without log.
expTrain = logRank(expTrain, base = 0)
# load in the constructed GRN
grn_all = utils_loadObject("grnAll.rda")
# load in constructed classifier. We use the gene pairs selected for classification to determine the weight of genes in the GRN status calculation
classyReturn = utils_loadObject("BroadClassifier_return.rda")
cnProc = classyReturn$cnProc
# extract the importance of genes based on the classifier
geneImportance = processImportance(classifier = cnProc$classifier, xpairs = classyReturn$xpairs_list, prune = TRUE)
# training normalization parameters
trainNormParam = ccn_trainNorm(expTrain, stTrain, subNets=grn_all$ctGRNs$geneLists, classList = geneImportance, dLevel = "project_id", sidCol = "barcode", classWeight = TRUE, exprWeight = FALSE, meanNorm = TRUE)
save(trainNormParam, file = "trainingNormalization.rda")
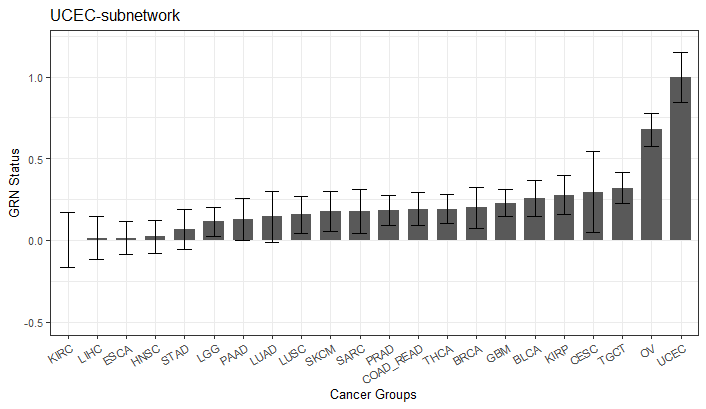
You can also visualize the GRN status of training samples.
# get the GRN status matrix
GRN_mean = trainNormParam$trainingScores
# select the GRN status for UCEC GRN
temp_mean = GRN_mean[GRN_mean$subNet == "TCGA-UCEC", ]
ggplot(data = temp_mean) +
geom_bar(stat="identity", data = temp_mean, aes(x=reorder(grp_name, mean), y=mean), width = 0.7) +
geom_errorbar(aes(ymin=mean - stdev, ymax = mean + stdev, x = grp_name), width = 0.5)+
ggtitle(paste0("TCGA-UCEC-subnetwork")) +
ylim(0, 1.2)+
xlab("Cancer Groups")+
ylab("GRN Status")+
#geom_hline(yintercept=1, linetype="dashed", color = "steelblue")+
theme_bw()+
theme(text = element_text(size=10),legend.position="none",axis.text.x = element_text(angle = 30, hjust = 1))
library(cancerCellNet)
# load in GRN network
grn_all = utils_loadObject("grnAll.rda")
# load in classifier
classReturn = utils_loadObject("BroadClassifier_return.rda")
# load in normalization parameters
trainNorm_param = utils_loadObject("trainingNormalization.rda")
iGenes = utils_loadObject("iGenes.rda")
CCL_samples = utils_loadObject("CCLE_UCEC.rda")
CCL_samples = CCL_samples[iGenes, ]
# rank the query sample genes
CCL_query = logRank(CCL_samples, base = 0)
GRN_statusQuery = ccn_queryGRNstatus(expQuery = CCL_query, grn_return = grn_all, trainNorm = trainNorm_param, classifier_return = classReturn, prune = TRUE)
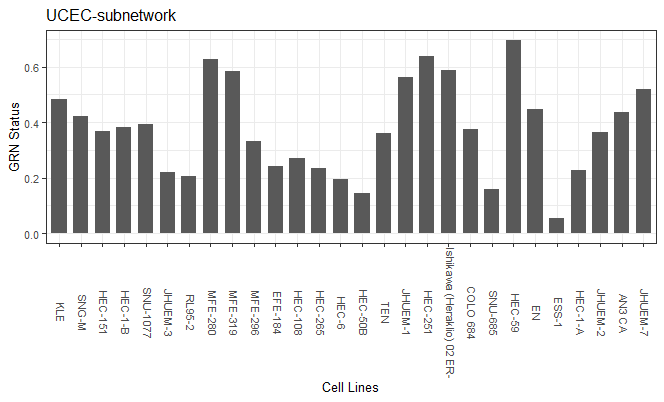
The output is a matrix with samples as column names and cancer types as row names. The values indicate the query sample's GRN status of in cancer specific subnetwork.
You can visualize GRN status of a cancer by
plotDf = data.frame("CellLines" = colnames(GRN_statusQuery),
"GRN_Status" = as.vector(GRN_statusQuery["UCEC", ]))
plotDf$CellLines <- factor(plotDf$CellLines, levels = plotDf$CellLines)
ggplot(data = plotDf) +
geom_bar(stat="identity", data = plotDf, aes(x=CellLines, y=GRN_Status), width = 0.7) +
ggtitle("UCEC-subnetwork") +
xlab("Cell Lines")+
ylab("GRN Status")+
#geom_hline(yintercept=1, linetype="dashed", color = "steelblue")+
theme_bw()+
theme(text = element_text(size=10),legend.position="none",axis.text.x = element_text(angle = 270, vjust=0.2))
TF scores are metric indicating the importance of transcription factors in establishing cancer type specific gene regulatory network. The magnitude of the score indicates the importance of the transcription factor. If the TF score is a positive number, it indicate that the TF should be more upregulated to have similar GRN to the desired cancer type. If the TF score is a negative number, it indicates that the TF should be downregulated to have similar GRN to the desired cancer type.
library(cancerCellNet)
# load in GRN network
grn_all = utils_loadObject("grnAll.rda")
# load in classifier
classReturn = utils_loadObject("BroadClassifier_return.rda")
# load in normalization parameters
trainNorm_param = utils_loadObject("trainingNormalization.rda")
iGenes = utils_loadObject("iGenes.rda")
CCL_samples = utils_loadObject("CCLE_UCEC.rda")
CCL_samples = CCL_samples[iGenes, ]
# rank the query sample genes
CCL_query = logRank(CCL_samples, base = 0)
GRN_statusQuery = ccn_queryGRNstatus(expQuery = CCL_query, grn_return = grn_all, trainNorm = trainNorm_param, classifier_return = classReturn, prune = TRUE)
The output should be a matrix with TF as row names and samples as column names.
In order to apply classifier on scRNA-seq profiles, you would have to aggregate the expression profiles within the same cluster label or cell type label. You can download a compiled sample scRNA expression profile and sample table to follow along with the demonstration. The compiled sample scRNA data were from Jerby-Arnon, L. et al. and Darmanis, S. et al.
scRNA_exp = utils_loadObject("sample_scRNA_exp.rda")
scRNA_st = utils_loadObject("sample_scRNA_st.rda")
agg_list = aggregate_scProfiles(scRNA_exp, scRNA_st, cell_id_col = "cell_id", group_id_col = "cell_type")
classMatrix_broad = broadClass_predict(broad_return$cnProc, agg_list$agg_exp, nrand = 2)
ccn_hmClass(classMatrix_broad, fontsize_col = 10, fontsize_row=10)