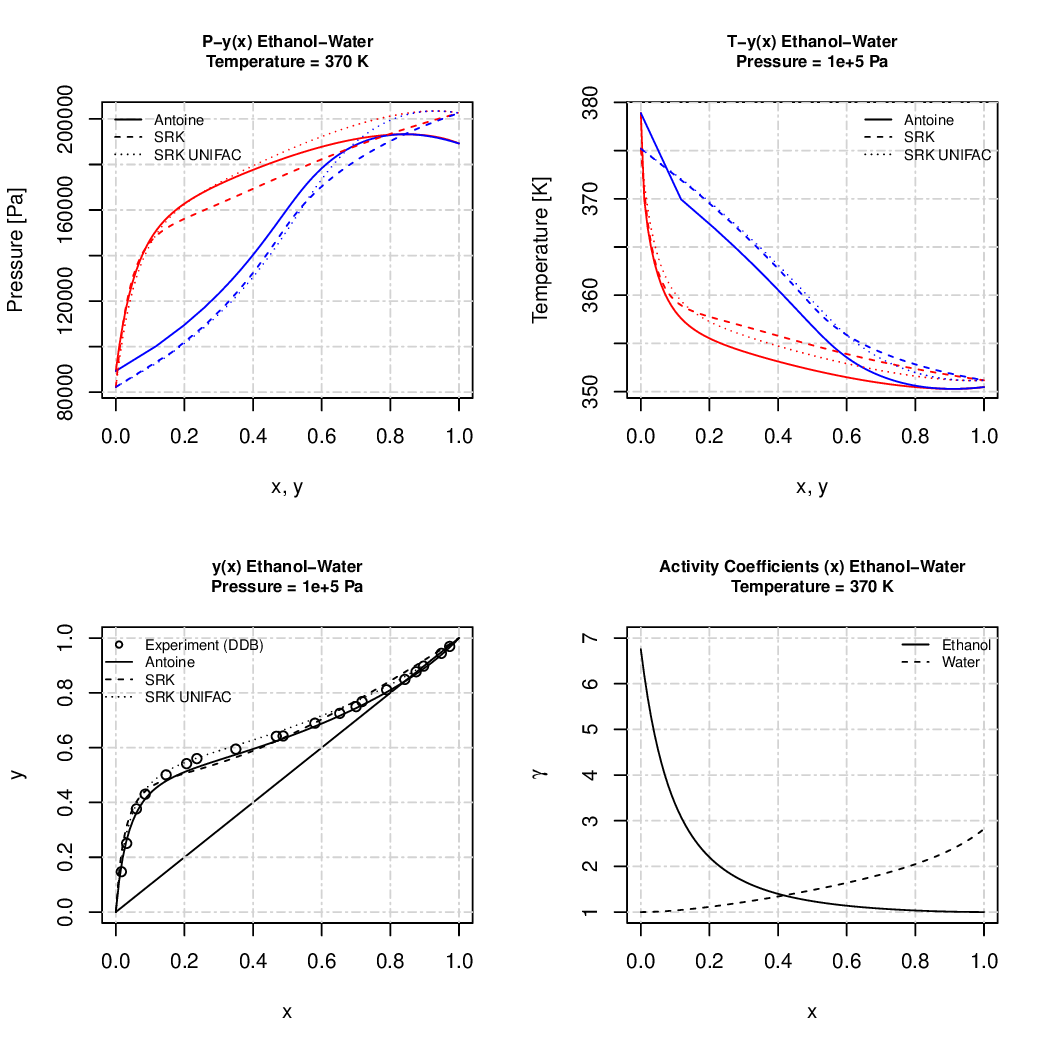
vapor liquid equilibrium calculations
This functions are part of a master thesis for blending multicomponent mixtures and simulate whose distillation curves.
The code is written in R script interpreter language for stastical mathematics. https://cran.r-project.org/
Bubble- and dewpoint calculations using Antoine and cubic equations with UNIFAC to fetch real mixture behavior.
All values are SI values (pressure [Pa], temperature [K]).
Examples
- UNIFAC
UNIFAC.gen(Substances=c('ethanol','water'), verbose=T) # returns surface (unu) and interaction parameter (aij) matrizes
UNIFAC(Fractions=c(0.4,0.6), unu=unu, aij=aij, Temperature=300) # returns activity coefficients
- Distillation curves
cs.boilingline(substances=c('ethanol', 'water'), fractions=c(0.4,0.6), pressure=1e+5, verbose=T) # returns
distillation curves for a closed system scenario, using two models
os.boilingline(substances=c('ethanol', 'water'), fractions=c(0.4,0.6), pressure=1e+5, verbose=T) # returns
distillation curves for a open system scenario, using five models
- Cubic Soave Redlich Kwong
3.1 Bubblepoint Temperature
SRK(temperature = 300, x=c(0.4,0.6), Tc=c(647.3, 562.0), Pc=c(221.20, 48.98), Ac=c(0.344,0.211), method = 'bubbleP')
Returns the bubblepoint pressure, gas fractions, fugacity coefficients and the departure functions of the enthalpy and entropy for the binary water-benzene mixture.