Software accompanying "HINGE: Long-Read Assembly Achieves Optimal Repeat Resolution"
-
Preprint: http://biorxiv.org/content/early/2016/08/01/062117
-
An ipython notebook to reproduce results in the paper can be found in this repository.
HINGE is a long read assembler based on an idea called hinging.
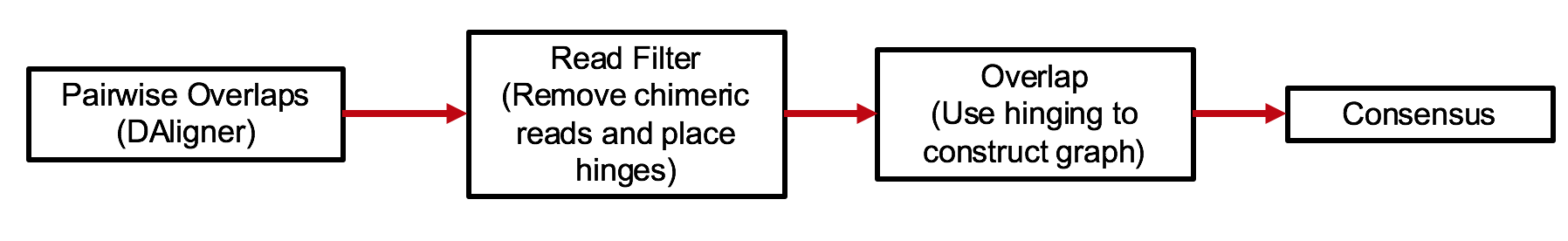
HINGE is an OLC(Overlap-Layout-Consensus) assembler. The idea of the pipeline is shown below.
At a high level, the algorithm can be thought of a variation of the classical greedy algorithm. The main difference with the greedy algorithm is that rather than each read having a single successor, and a single predecessor, we allow a small subset of reads to have a higher number of successors/predecessors. This subset is identified by a process called hinging. This helps us to recover the graph structure directly during assembly.
Another significant difference from HGAP or Falcon pipeline is that it does not have a pre-assembly or read correction step.
Reads filtering filters reads that have long chimer in the middle, and short reads.
Reads which can have higher number of predecessors/successors are also identified there.
This is implemented in filter/filter.cpp
The layout is implemented in layout/hinging.cpp. It is done by a variant of the greedy algorithm.
The graph output by the layout stage is post-processed by running scripts/pruning_and_clipping.py.
One output is a graphml file which is the graph representation of the backbone.
This removes dead ends and Z-structures from the graph enabling easy condensation.
It can be analyzed and visualized, etc.
In the pipeline described above, several programs load their parameters from a configuration file in the ini format. All tunable parameters are described in this document.
- g++ 4.8
- cmake 3.x
- libhdf5
- boost
- Python 2.7
The following python packages are necessary:
- numpy
- ujson
- configparser
- colormap
- easydev.tools
- pbcore
This software is still at prototype stage so it is not well packaged, however it is designed in a modular flavor so different combinations of methods can be tested.
Installing the software is very easy.
git clone https://github.com/fxia22/HINGE.git
git submodule init
git submodule update
./utils/build.sh
Alternatively, you can use docker to build and use HINGE, see this guide for more information.
In order to call the programs from anywhere, I suggest one export the directory of binary file to system environment, you can do that by using the script setup.sh. The parameters are initialised in utils/nominal.ini. The path to nominal.ini has to be specified to run the scripts.
A demo run for assembling the ecoli genome is the following:
source utils/setup.sh
mkdir data/ecoli
cd data/ecoli
# reads.fasta should be in data/ecoli
fasta2DB ecoli reads.fasta
DBsplit -x500 -s100 ecoli
HPC.daligner -t5 ecoli | csh -v
# alternatively, you can put output of HPC.daligner to a bash file and edit it to support
rm ecoli.*.ecoli.*
LAmerge ecoli.las ecoli.+([[:digit:]]).las
rm ecoli.*.las # we only need ecoli.las
DASqv -c100 ecoli ecoli.las
# Run filter
mkdir log
hinge filter --db ecoli --las ecoli.las -x ecoli --config <path-to-nominal.ini>
# Get maximal reads
hinge maximal --db ecoli --las ecoli.las -x ecoli --config <path-to-nominal.ini>
# Run layout
hinge layout --db ecoli --las ecoli.las -x ecoli --config <path-to-nominal.ini> -o ecoli
# Run postprocessing
hinge clip ecoli.edges.hinges ecoli.hinge.list <identifier-of-run>
# get draft assembly
hinge draft-path <working directory> ecoli ecoli<identifier-of-run>.G2.graphml
hinge draft --db ecoli --las ecoli.las --prefix ecoli --config <path-to-nominal.ini> --out ecoli.draft
# get consensus assembly
hinge correct-head ecoli.draft.fasta ecoli.draft.pb.fasta draft_map.txt
fasta2DB draft ecoli.draft.pb.fasta
HPC.daligner ecoli draft | zsh -v
hinge consensus draft ecoli draft.ecoli.las ecoli.consensus.fasta <path-to-nominal.ini>
hinge gfa <working directory> ecoli ecoli.consensus.fasta
#results should be in ecoli_consensus.gfa
Some programs are for debugging and oberservation. For example, one can get the ground truth by mapping reads to reference and get ecoli.ecoli.ref.las.
This las file can be parsed to json file for other programs to use.
run_mapping.py ecoli ecoli.ref ecoli.ecoli.ref.las 1-$
In the prune step, if ecoli.mapping.json exists, the output graphml file will contain the information of ground truth.
Draw a read, for example 60947, and output figure to sample folder (need plus 1 as LAshow counts from 1):
draw2.py ecoli ecoli.las 60948 sample 100
Draw pileup on draft assembly, given a region(start,end):
draw2_pileup_region.py 3600000 4500000
For ecoli 160X dataset, after shortening reads to have a mean length of 3500 (with a variance of 1500), the graph is preserved.
Results on the bacterial genomes of the NCTC 3000 project can be found at web.stanford.edu/~gkamath/NCTC/report.html