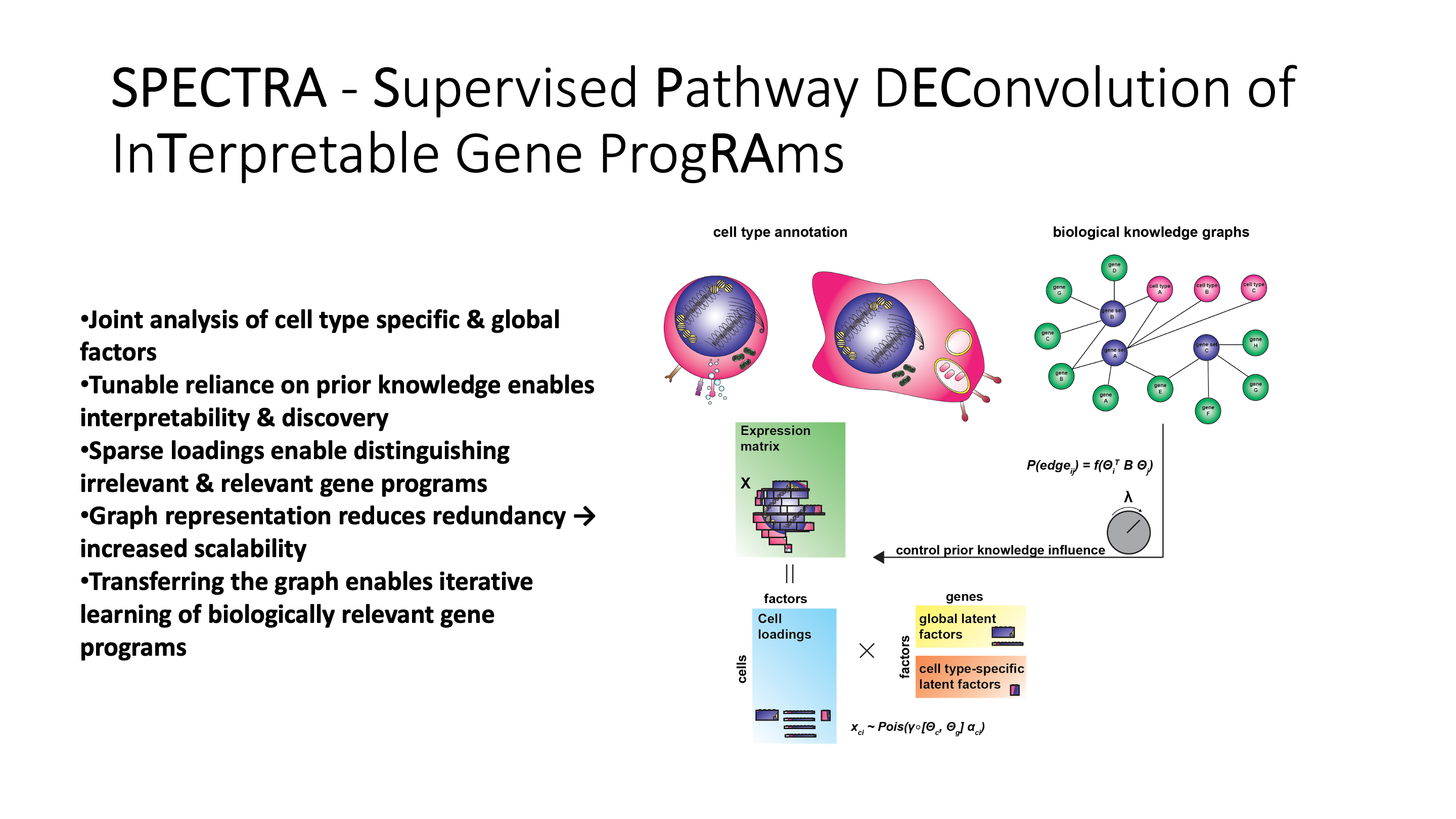
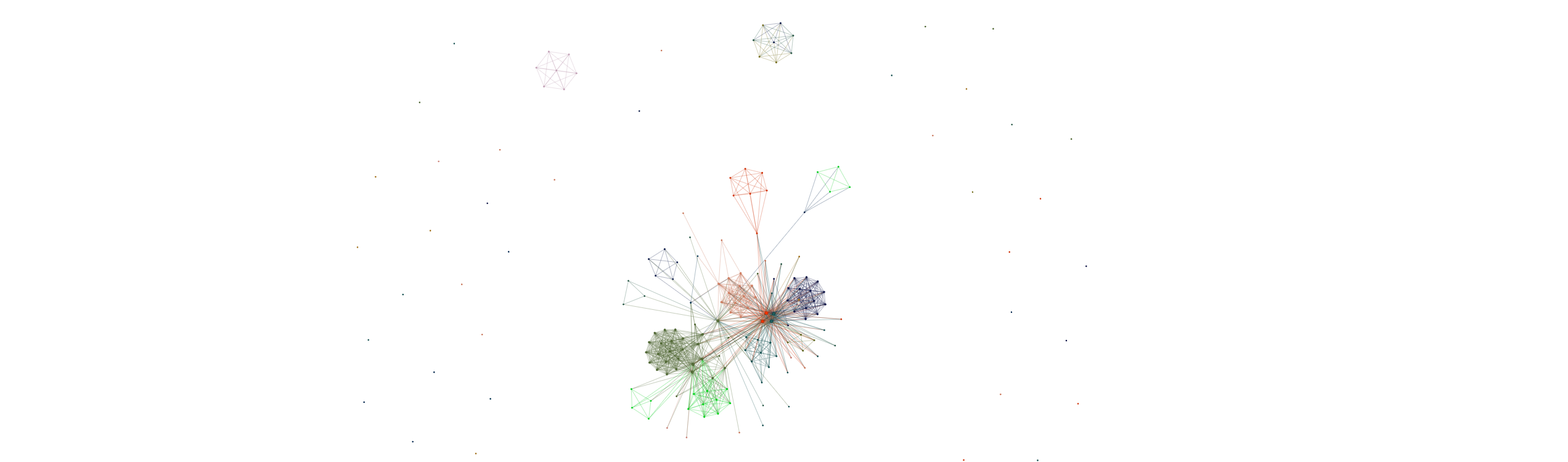
SPECTRA takes in a single cell gene expression matrix and a set of pathway annotations to fit the data to.
gene_set_annotations = {
"global": {"global_ifn_II_response" : ["CIITA", "CXCL10", ...] , "global_MHCI": ["HLA-A". "HLA-B", "HLA-C"] },
"CD8_T": {"CD8_exhaustion" : ["CXCL13", "LAG3", "CD39", ...]}
...
}
Alternatively, SPECTRA can take in a set of adjacency matrices representing networks for each cell type:
gene_set_annotations = {
"global": adjacency_matrix1,
"CD8_T": adjacency_matrix2,
...
}
A pypi package will be available soon. For installation, you can add spectra to pip:
git clone https://github.com/dpeerlab/spectra
cd spectra
pip install .
Data used for the examples can be found here: dropbox link to h5ad file . This is a subsetted version of the Bassez et al. Nature Medicine (https://doi.org/10.1038/s41591-021-01323-8) preprocessed by scran and emptydrops with annotated cell types at various resolutions.
We start by importing spectra. The easiest way to run spectra is to use the est_spectra function in the spectra module, as shown below. The default behavior is to set the number of factors equal to the number of gene sets plus one. However, this can be modified by passing an integer e.g. L = 20 as an argument to the function or a dictionary that maps cell type to an integer per cell type. We provide a method for estimating the number of factors directly from the data by bulk eigenvalue matching analysis, which is detailed further below.
from spectra import spectra as spc
model = spc.est_spectra(adata = adata, gene_set_dictionary = gene_set_dictionary, cell_type_key = "cell_type", use_highly_variable = True, lam = 0.01)
This function stores four important quantities in the AnnData, in addition to returning a fitted model object. Factors are the scores that tell you how much each gene contributes to each factor, while markers is an array of genes with top scores for every factor. Cell scores are similarly the score of each factor for every cell. Finally, vocab is a boolean array that is True for genes that were used while fitting the model - note that this quantity is only added to the AnnData when highly_variable is set to True
factors = adata.uns['SPECTRA_factors'] # factors x genes matrix that tells you how important each gene is to the resulting factors
markers = adata.uns['SPECTRA_markers'] # factors x K [where K is a user specified number] list of K top markers per factor
cell_scores = adata.obsm['SPECTRA_cell_scores'] # cells x factors matrix of cell scores
vocab = adata.var['spectra_vocab'] # boolean matrix of size # of genes that indicates the set of genes used to fit spectra
To access finer grained information about the model fit, we can look at the attributes of the model object directly. Model parameters can be accessed with functions associated with the model object
model.return_eta_diag()
model.return_cell_scores()
model.return_factors()
model.return_eta()
model.return_rho()
model.return_kappa()
model.return_gene_scalings()
Apart from cell scores and factors, we can also retrive a number of other parameters this way that are not by default added to the AnnData. Eta diag is the diagonal of the fitted factor-factor interaction matrix; however, its interpretation is that it measures the extent to which each factor is influenced by the prior information. In practice many of these values are zero, indicating that they are estimated without bias introduced by the annotation set. Eta is the full set of factor-factor interaction matrices, whose off diagonals measure the extent to which factors share the same genes. Rho and kappa are parameters that control the background rate of non-edges and edges respectively. These can be fixed throughout training (default) or estimated from the data by providing rho = None or kappa = None to the est_spectra() function or to model.train(). Finally gene scalings are correction factors that normalize each gene based on its mean expression value.
To interpret factor weight based marker lists with respect to the input annotations, we take the overlap coefficient with the input sets. The spectra model object has a function to do this matching(markers, annotations,threshold)
model.matching(adata.uns["SPECTRA_markers"], annotations, threshold = 0.4)
One outcome of fitting spectra is to fit a gene-gene graph where edges represent similarities between latent variables associated with each gene (a smoothed version of transcriptional similarity) To access this for say, TNK cells; use the following
soft_graph = model.return_graph(ct = "TNK")
for large numbers of genes its clumsy to visualize the whole graph - to visualize a subgraph formed around a particular list of genes, use:
out = spc.graph_network(adata, soft_graph, gene_set)
out.show("test_graph.html")
this will take N closest genes to your gene set and only visualize this subgraph. The interactive graph file gets saved as an html. To visualize multiple gene sets at the same time, we have a different version of the function that assigns a random color to each gene set:
#gene sets is a list of lists
out = spc.graph_network_multiple(adata,soft_graph, gene_sets)
out.show("test_graph.html")
#save trained model
model.save("test_model")
#initialize a model and load trained model, adata must be have the attributes stored by est_spectra
model = spc.load_from_pickle("test_model", adata, gene_set_dictionary, cell_type_key)
Instead of passing an AnnData object to est_spectra one can pass np.ndarray objects directly. The **kwargs contains arguments to the training function, lr_schedule = [1.0,.5,.1,.01,.001,.0001],num_epochs = 10000, verbose = False. To do this, initialize a model:
model = spc.SPECTRA_Model(X = X, labels = labels, L = L, vocab = vocab, gs_dict = gene_set_dictionary, use_weights = use_weights, lam = lam, delta=delta,kappa = kappa, rho = rho, use_cell_types = use_cell_types)
model.train(X = X, labels = labels,**kwargs)
It is also required to run the model this way if you want to input arbitrary adjacency matrices instead of a dictionary of gene sets. The gene set dictionary is used to create an adjacency matrix when it is not None.
model = spc.SPECTRA_Model(X = X, labels = labels, L = L, adj_matrix = adj_matrix, weights = weights, lam = lam, delta=delta,kappa = kappa, rho = rho, use_cell_types = use_cell_types)
model.train(X = X, labels = labels,**kwargs)
#instead of training, load a pretrained model
model = spc.SPECTRA_Model(X = X, labels = labels, L = L, adj_matrix = adj_matrix, weights = weights, lam = lam, delta=delta,kappa = kappa, rho = rho, use_cell_types = use_cell_types)
model.load("test_model",labels = labels)
To estimate the number of factors first, run
from spectra import K_est as kst
L = kst.estimate_L(adata, attribute = "cell_type", highly_variable = True)
For smaller problems we can use a memory intensive EM algorithm instead
X = adata.X
A = binary adjacency matrix
model = spc.SPECTRA_EM(X = X, A= A, T = 4)
model.fit()
model.internal_model
SPECTRA(
(theta): ParameterDict(
(global): Parameter containing: [torch.FloatTensor of size 4324x20]
(B): Parameter containing: [torch.FloatTensor of size 4324x31]
(M): Parameter containing: [torch.FloatTensor of size 4324x15]
(Neutrophil): Parameter containing: [torch.FloatTensor of size 4324x3]
(TNK): Parameter containing: [torch.FloatTensor of size 4324x13]
)
(alpha): ParameterDict(
(B): Parameter containing: [torch.FloatTensor of size 6762x51]
(M): Parameter containing: [torch.FloatTensor of size 29328x35]
(Neutrophil): Parameter containing: [torch.FloatTensor of size 3492x23]
(TNK): Parameter containing: [torch.FloatTensor of size 77495x33]
)
(eta): ParameterDict(
(global): Parameter containing: [torch.FloatTensor of size 20x20]
(B): Parameter containing: [torch.FloatTensor of size 31x31]
(M): Parameter containing: [torch.FloatTensor of size 15x15]
(Neutrophil): Parameter containing: [torch.FloatTensor of size 3x3]
(TNK): Parameter containing: [torch.FloatTensor of size 13x13]
)
(gene_scaling): ParameterDict(
(global): Parameter containing: [torch.FloatTensor of size 4324]
(B): Parameter containing: [torch.FloatTensor of size 4324]
(M): Parameter containing: [torch.FloatTensor of size 4324]
(Neutrophil): Parameter containing: [torch.FloatTensor of size 4324]
(TNK): Parameter containing: [torch.FloatTensor of size 4324]
)
)