by Alec Pankow and Ben Murrell, now maintained by Hugh Murrell
now upgraded to Julia version 1.7.1
- first update all apps
apt updateapt upgrade
- Snakemake
apt-get install -y snakemake
- mafft
apt-get install -y mafft
- fasttree
apt-get install -y fasttree
- python3 packages
apt-get install python3-pandasapt-get install python3-seaborn
Download and unpack the latest Julia (we recommend version 1.7.1) from:
https://julialang.org/downloads/
Make sure you can enter the julia REPL from the command line, on an ubuntu machine you would do:
# move the julia system to a lib directory
mv julia-1.7.1 /usr/lib/julia-1.7.1
# make julia v1.7.1 executable from the command line
ln -s /usr/lib/julia-1.7.1/bin/julia /usr/local/bin/julia
# check that you can enter the julia REPL
julia --versionNow that the dependencies are setup we clone the PORPIDpipeline repository
cd ~
git clone git@github.com:MurrellGroup/PORPIDpipeline.gitthen navigate to the PORPIDpipeline project folder and start the Julia REPL.
Enter the package manager using ] and then enter
activate .
instantiate
precompileThis will activate, install, and precompile the julia environment specified by the
Project.toml and Manifest.toml files. The precompile command
above is not strictly needed but is useful if there are issues with installing
the julia packages listed in Project.toml
Next, add the following text to your Julia startup file (typically at ~/.julia/config/startup.jl;
you may need to create the directory if not present, mkdir -p ~/.julia/config).
using Pkg
if isfile("Project.toml") && isfile("Manifest.toml")
Pkg.activate(".")
endThis will activate the local environment at Julia startup.
To configure the PORPIDpipeline workflow, first edit the demo config.yaml file to reflect
your library construction.
It should follow the same format shown in the demo example below.
demo:
donor_1_REN:
cDNA_primer: CCGCTCCGTCCGACGACTCACTATAacagtgNNNNNNNNGTCATTGGTCTTAAAGGTACCTG
sec_str_primer: TAGGCATCTCCT
panel: "panels/HIV1_COM_2017_5970-8994_DNA_stripped.fasta"
donor_2_REN:
cDNA_primer: CCGCTCCGTCCGACGACTCACTATAcactcaNNNNNNNNGTCATTGGTCTTAAAGGTACCTG
sec_str_primer: TAGGCATCTCCT
panel: "panels/HIV1_COM_2017_5970-8994_DNA_stripped.fasta"
donor_3_REN:
cDNA_primer: CCGCTCCGTCCGACGACTCACTATAggtagcNNNNNNNNGTCATTGGTCTTAAAGGTACCTG
sec_str_primer: TAGGCATCTCCT
panel: "panels/HIV1_COM_2017_5970-8994_DNA_stripped.fasta"
donor_1_GP:
cDNA_primer: CCGCTCCGTCCGACGACTCACTATAacagtgNNNNNNNNGTATGTCATTGACAGTCCAGC
sec_str_primer: TTGACTAGCGGAGGCTAGAAGGAGA
panel: "panels/HIV1_COM_2017_787-3300_DNA_stripped.fasta"
donor_2_GP:
cDNA_primer: CCGCTCCGTCCGACGACTCACTATAcactcaNNNNNNNNGTATGTCATTGACAGTCCAGC
sec_str_primer: TTGACTAGCGGAGGCTAGAAGGAGA
panel: "panels/HIV1_COM_2017_787-3300_DNA_stripped.fasta"
donor_3_GP:
cDNA_primer: CCGCTCCGTCCGACGACTCACTATAggtagcNNNNNNNNGTATGTCATTGACAGTCCAGC
sec_str_primer: TTGACTAGCGGAGGCTAGAAGGAGA
panel: "panels/HIV1_COM_2017_787-3300_DNA_stripped.fasta"Note that the donor ID barcode is in lowercase and the Unique Molecular Identifier (UMI) barcode is indicated with N's. The primer sequences provided will be used for demultiplexing and will be trimmed from the final sequences.
The panel arg should be a path to a .fasta alignment spanning your amplicon,
with all gaps stripped. This will be used only in the postproccessing step to remove
off-target seqs and trim to the correct coordinates.
To generate your own panel file you are encouraged to visit:
https://www.hiv.lanl.gov/content/sequence/NEWALIGN/align.html
where you can download an alignment and then use aliview to trim
the alignment to your region of interest.
gzipped CCS .fastq files should be placed in the raw-reads/ subdirectory and named
according to the the dataset name used in the config.yaml file, ie, demo.fastq.gz
for the demo dataset.
Preview jobs with Snakemake and run with {n} cores.
#preview jobs
snakemake -np
#run
snakemake -j{n}For more info on Snakemake, see https://snakemake.readthedocs.io/en/stable/
Some (without root access) may prefer to setup PORPIDpipeline in a conda environment.
To accomplish this, first install anaconda locally. (the install script allows you to choose
the location for anaconda, by default /home/user but choose something else if
you want something accessable to a group of users)
curl –O https://repo.anaconda.com/archive/Anaconda3-2021.05-Linux-x86_64.sh > Anaconda3-2021.05-Linux-x86_64.sh
bash Anaconda3-2021.05-Linux-x86_64.shthen log out and log in again and check that you are in the base environment.
conda is very slow, so we suggest installing mamba in the conda base environment:
conda install -n base -c conda-forge mambaclone the PORPIDpipeline repository
cd ~ # or some other directory used for your anaconda installation
git clone git@github.com:MurrellGroup/PORPIDpipeline.gitand then all the PORPIDpipeline dependencies including julia version 1.7.1
( as listed in the PORPIDpipeline conda environment spec in environment.yaml),
can be installed in a conda environment via mamba using the commands:
conda config --add channels conda-forge
conda config --add channels bioconda
mamba env create --file environment.yamlNote that if you did use some other directory than your home directory for
installing the PORPIDpipeline repository then you have to inform Julia where
your packages are stored by placing the following command in your .bashrc
file:
# set path to .julia files
export JULIA_DEPOT_PATH="/some/other/directory/.julia"to complete the setup, activate the new PORPIDpipeline conda environment,
conda activate PORPIDpipelineand continue with the julia package environment setup as outlined above in the quick start section.
Seting up a snakemake pipeline on a cluster is a dark art. Here we describe an attempt
at installing PORPIDpipeline on a two node cluster, (one node a controller node with 16 cores
and the other node a compute node with 64 cores).
Firstly, since the cluster administrator is hardly likely to give you root access we
suggest you follow the conda installation for PORPIDpipeline. If you expect more
than one user of your PORPIDpipeline then install in a directory that all
your users can see and that is visible from both the contoller and compute nodes.
ie use some other directory rather than the standard home directory and make
sure to inform julia about this choice of directory as
outlined in the conda section above.
Secondly, cluster administrators usually insist that large data sets are stored
in an appropriate volume and not in the usual user's space. On our cluster the
administrator required the PORPIDpipeline code to be installed in a \tools\porpid\
directory and the large data sets (input, output and temporary) to be stored in
a \data\porpid\ directory so we installed PORPIDpipeline into \tools\porpid\porpidpipeline
and then replaced some of the directories in the porpidpipeline
directory with symbolic links to an appropriate directory in the \data\porpid\ directory
as shown below
config.yaml -> /raw/porpid/config/demo.yaml
panels -> /raw/porpid/panels/
porpid -> /raw/porpid/porpid/
postproc -> /raw/porpid/postproc/
raw-reads -> /raw/porpid/raw-reads/
Naturally, one must copy contents of the installation to the /raw/porpid/ directory
before deleting the installation directory and replacing it with a symbolic link to the
appropriate place on the raw volume.
Job submission, after setting up like this we are ready to run the demo study through
the PORPIDpipeline
by submitting the snakemake command to the cluster managemant system.
On our cluster that management system is slurm and the following shell script
stored in porpid_job.sh facilitated that submission:
#!/bin/bash
#SBATCH --job-name==porpid
#SBATCH --time=1:0:0
#SBATCH --nodes=1
#SBATCH --ntasks-per-node=7
#SBATCH --partition=main
if [ "$#" -lt 1 ]; then
echo "please supply a config file name as first parameter"
exit
fi
echo "config file is $1"
echo "${SLURM_JOB_NAME} job submited using ${SLURM_NTASKS} cores"
# create a symbolic link for the snakemake config file to point to the config for the current study
rm -f /tools/PORPIDpipeline/porpidpipeline/config.yaml
ln -s /RAW/PORPID/CONFIG/$1.yaml /tools/PORPIDpipeline/porpidpipeline/config.yaml
# tell slurm where anaconda is and conda activate the PORPIDpipeline environment
source /tools/PORPIDpipeline/anaconda3/etc/profile.d/conda.sh
conda activate PORPIDpipeline
# navigate to the porpidpipeline directory and run snakemake
# add -F to to the snakemake command to force re-run of all rules
cd /tools/PORPIDpipeline/porpidpipeline
snakemake --rerun-incomplete -j${SLURM_NTASKS} To submit the demo to run as a slurm batch job one just uses
sbatch porpid_job.sh demoThe script above sets some environment variables for slurm and then resets
the symbolic link to the appropriate config file for the demo study.
It then activates the conda environment switches to the installation
directory and runs the snakemake pipeline.
With this structure it is easy to run a new study through PORPIDpipeline.
One copies the new config file into the /raw/porpid/config/ directory,
transfers the fastq data to the /raw/porpid/raw-reads/ directory
and then issues the sbatch command using the appropriate study name
instead of demo
Note that with this method you must predetermine the number of cores
you intend to use on your cluster's node. In the demo study this is set
to 7 ( 6 cores for the samples to run in parallel plus 1 core for snakemake )
Each study will be different. To see how many samples can be run in parallel
you can do a snakemake dry run using the porpid_dry_run.sh script below:
#!/bin/bash
if [ "$#" -lt 1 ]; then
echo "please supply a config file name as first parameter"
exit
fi
echo "config file is $1"
# create a symbolic link for the snakemake config file to
# point to the config for the current study
rm -f /tools/PORPIDpipeline/porpidpipeline/config.yaml
ln -s /RAW/PORPID/CONFIG/$1.yaml /tools/PORPIDpipeline/porpidpipeline/config.yaml
# activate the conda environment
source /tools/PORPIDpipeline/anaconda3/etc/profile.d/conda.sh
conda activate PORPIDpipeline
# perform a snakemake dry run
# remove the -f for a partial dry run of what's left to do
cd /tools/PORPIDpipeline/porpidpipeline
snakemake -F --rerun-incomplete -npNote that this dry run is not compute intensive and can ve executed on the
controller machine without using the sbatch command as follows:
./porpid_dry_run.sh demoThe above suggestion for running a snakemake pipeline under slurm
is rudamentary. Maximum cores must be requested at the start of execution
and they are probably held throughout the run.
However, it is alledged that snakemake can play nicely with slurm and
it should be possible to have snakemake invoke slurm for each rule in
the pipeline. In this case snakemake would request the optimal number
of cores needed for each step in the pipeline.
We have not attempted this yet, and it would probably require writing a
slurm efficient version of the snakefile.
Watch this space for further developments.
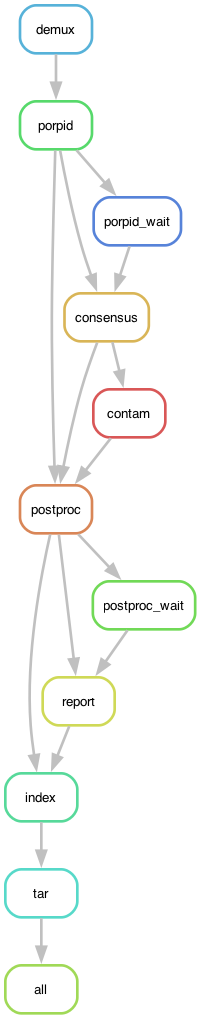
The graph below summarizes the overall organization of the workflow. Each node in the graph is a rule in the The Snakefile.
An introduction to PacBio sequencing and an explanation for each PORPIDpipeline rule is given in the set of introductory slides packaged with this repository. docs/slides/PORPIDpipeline.pdf