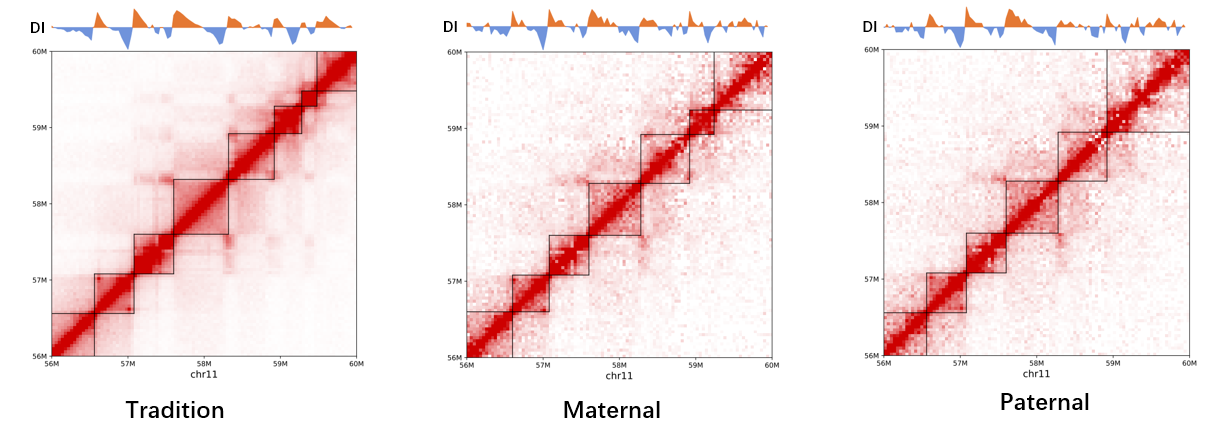
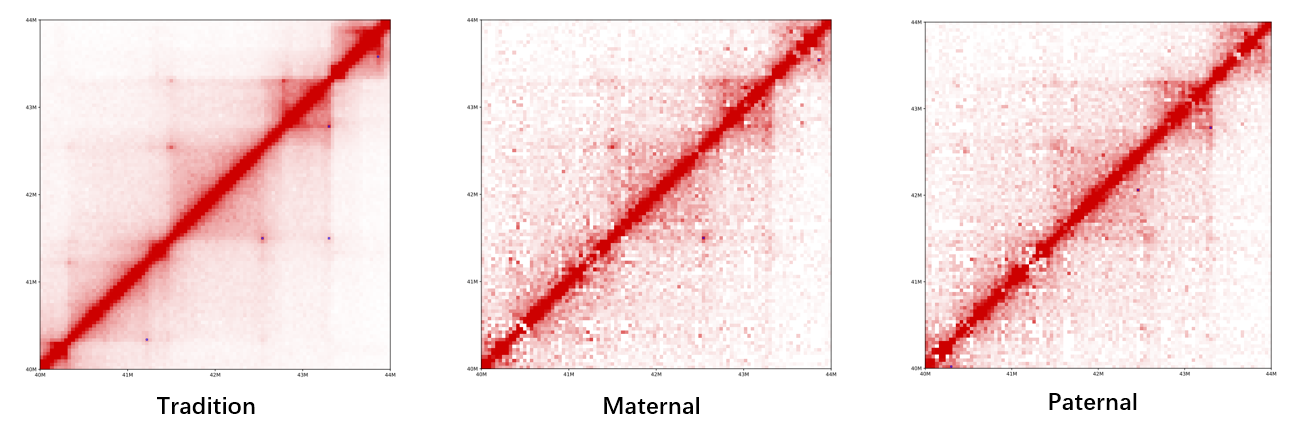
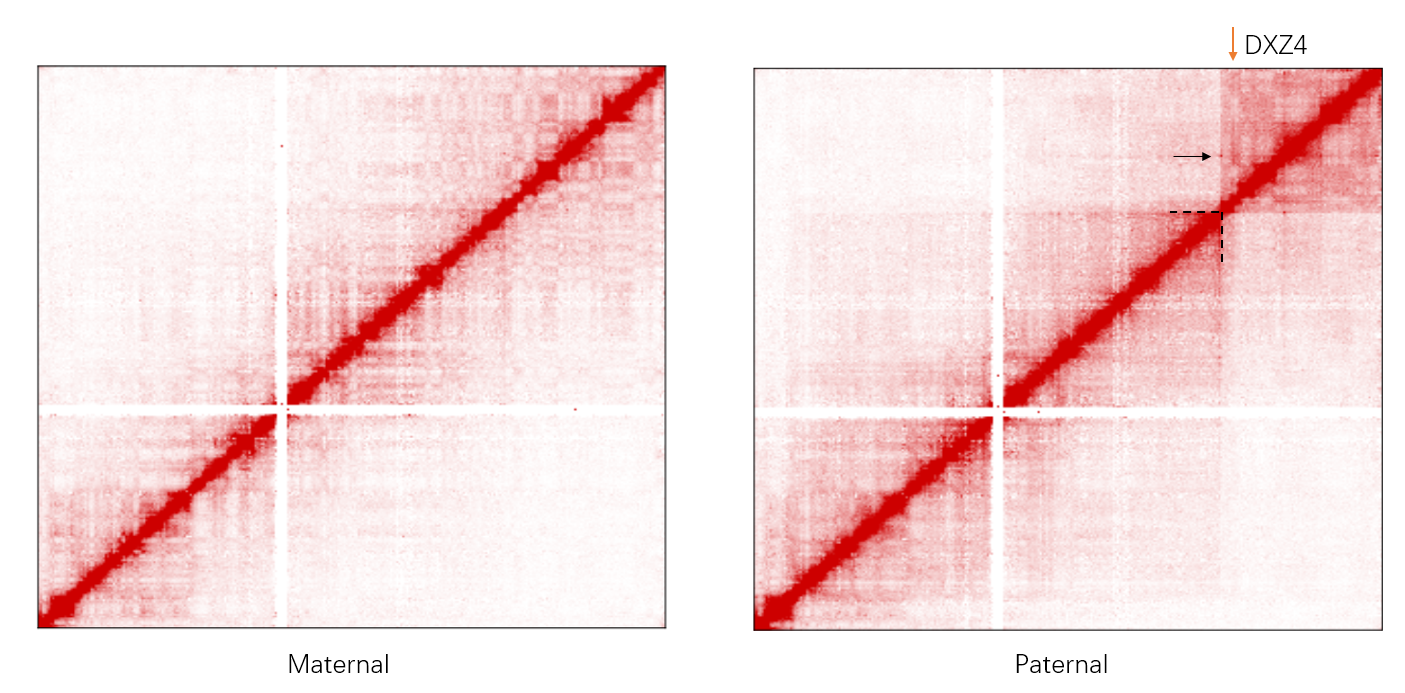
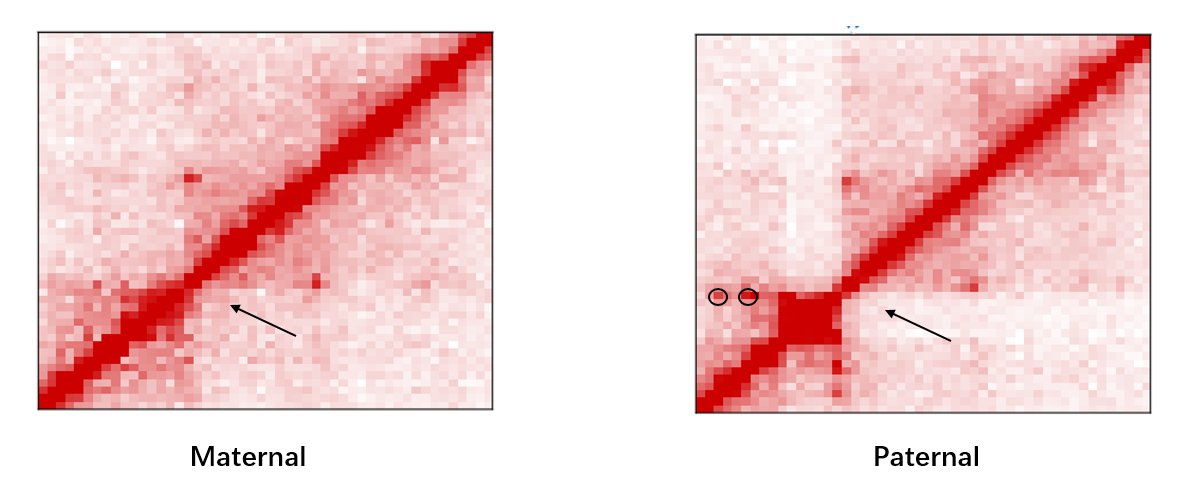
HiCHap is a Python package designed to process and analyze Hi-C data, primarily for diploid Hi-C by using phased SNPs. First, the Hi-C reads are split in ligation junction sites, and then all split parts are used in mapping to maximumly utilize SNPs in allele assignment, thus improving the ratios of allele-assigned reads. The noisy reads are further eliminated. Second, except for traditional data bias caused by Hi-C experiments, the unevenly distributed genetic variants lead to additional bias in reconstructed Hi-C haplotype because it is potentially easier to assign allelic contacts in the chromatin regions with denser genetic variants. HiCHap utilizes a two-step strategy to reduce these two types of data biases by using the mapped and allele-assigned contacts only. Third, with the improved quality of reconstructed Hi-C haplotype, HiCHap can identify compartments, topological domains/boundaries and chromatin loops at haplotype level, and also provide testing on the allelic specificity for these structures. Finally, HiCHap also supports data processing, bias correction and structural analysis for traditional Hi-C without separating homologous chromosomes.
Download :
PyPI : (https://pypi.org/project/HiCHap/)
Github : (https://github.com/Prayforhanluo/HiCHap_master)
Luo, H., Li, X., Fu, H. et al. HiCHap: a package to correct and analyze the diploid Hi-C data. BMC Genomics 21, 746 (2020). https://doi.org/10.1186/s12864-020-07165-x
- Python 2.7+
- Multiprocess
- Numpy
- Scipy
- statsmodels
- Scikit-Learn
- xml
- pysam
- ghmm
- Bio
- bowtie2 (we used 2.2.9)
- samtools (we used 1.5)
- cooler
Please use conda , pip or the source code to install them if necessary
Download the HiCHap source code from PyPI or Github, unzip the files and run the setup.py script::
$ python setup.py install
Or use the pip install
$ pip install HiCHap
By the way, When I developed HiCHap, I have found a strange bug. when HiCHap has been installed, I use the hichap -h to get help information. But it bugs with "pkg_resources.ResolutionError::". I just figure out how to fix it but i can't figure out why it happens. The fixing code is in the setup.py at the last few lines. If anyone can tell me how to avoid this bug. Please connect with me (hluo_lc@outlook.com) and thanks so much :)
HiCHap will be installed successfully if no exception occurs in the above process.
Hi-C data format is fastq.gz(or unzipped fastq), and canonical name will be nice, for example::
$ ls -lh
-rw-r--r-- 1 hluo CPeng 42G Sep 14 00:30 GM12878_R1_1.fastq.gz
-rw-r--r-- 1 hluo CPeng 42G Sep 14 00:30 GM12878_R1_2.fastq.gz
Both compresssed and uncompressed fastq data are available for Hi-C data
The genome data format is .fa. Please use the same reference genome version when processing different data, such as phased genetic variants and allelic read mapping..
$ ls -lh
-rwxr-xr-- 1 hluo CPeng 2780868912 Sep 4 2017 mm10.fa
-rwxr-xr-- 1 hluo CPeng 3187102198 Sep 4 2017 hg19.fa
The phased SNPs can be given in a TXT-like file. The file has the following five columns :
- chromosome ID
- genome position
- reference base
- Maternal base
- Paternal base
For example::
$ ls -lh
-rw-r--r-- 1 hluo CPeng 56524229 Mar 9 11:07 GM12878_F1_maternal_paternal_SNP.txt
$ head -5 GM12878_F1_maternal_paternal_SNP.txt
1 10492 C T C
1 10927 A A G
1 10938 G G A
1 13302 C C T
1 13813 T G T
First, try to get the help message! The executable code in the command line of HiCHap is hichap.
HiCHap has the general parameters : -w (--workspace), -log (--logfile) and -N (--NonAllelic).
Specifically, hichap will output the results to the current workspace (-w) and search the input there. Users can also set the workspace by themselves. If -N (--NonAllelic) is set on the command, this sub-module will run the traditional Hi-C pipeline.
If -N (--NonAllelic) is set on the sub-command. the sub-module will running traditional Hi-C pipeline.
Try to get help information:
$ hichap --help
usage: hichap [-h] [-v] {rebuildG,rebuildF,GlobalMapping,Rescue,ReMapping,bamProcess,filtering,matrix}
...
...
There are several major functions available in HiCHap sub-commands.
| Subcommand | Description |
|---|---|
| rebuildG | Build Genome index, genome size and enzyme fragment locations |
| rebuildF | Chunk fastq files. |
| GlobalMapping | Mapping the raw chunked reads to genome |
| Rescue | Rescue the unmapped reads by cutting the Ligation site. |
| ReMapping | Re-mapping the rescued reads to genome |
| bamProcess | Integrate all the mapping information |
| filtering | Hi-C filtering and Allelic assignment (if necessary). |
| matrix | Interaction Matrix Construction |
Please use 'hichap SUB-COMMAND -h' to see the detail description for each option of each module.
For traditional Hi-C pipeline, build the genome index and enzyme-fragment locations for the reference genome.
The command line is ::
$ hichap rebuildG -w ./GM12878 -log ./GM12878.log -N -g ./hg19.fa -e MboI -t 4
For Hi-C haplotype pipeline, build the maternal and/or paternal genomes and corresponding indexes, enzyme-fragment locations.
The command line is ::
$ hichap rebuildG -w ./GM12878 -log ./GM12878.log -g ./hg19.fa -S ./GM12878_F1_maternal_paternal_SNP.txt -e MboI -t 4
After subcommand rebuildG, a folder (./GM12878/genome) containing the genome indexes and the txt files containing fragment locations will be created under the workspace(./GM12878)
Chunk the fastq by a given step. The command line eg ::
$ hichap rebuildF -w ./GM12878 -log ./GM12878.log -1 GM12878_R1_1.fastq.gz -2 GM12878_R1_2.fastq.gz -c 4000000 -t 2
After rebuildF, a folder(./Genome/fastqchunks) contains the chunked files will be created under the workspace(./Genome) eg:
$ ls -lh ./Genome/fastqchunks
-rw-r--r-- 1 hluo CPeng 410M Nov 13 10:44 GM12878_R1_chunk0_1.fastq.gz
-rw-r--r-- 1 hluo CPeng 410M Nov 13 10:43 GM12878_R1_chunk0_2.fastq.gz
...
...
-rw-r--r-- 1 hluo CPeng 407M Nov 13 10:49 GM12878_R1_chunk9_1.fastq.gz
-rw-r--r-- 1 hluo CPeng 406M Nov 13 10:48 GM12878_R22_chunk9_2.fastq.gz
After genome rebuilding and fastq chunking, you need to start mapping tasks. Each chunk represents a single mapping task. The parallel mode is used to reduce the time cost. And two sets of mapping APIs are designed for different computer environments.
If you use the clusters (based on PBS for jobs management), you can choose the PBS API. You can submit N tasks to the computing nodes and M threads for each task.
For traditional Hi-C pipeline, there is only one index parameter. For example:
$ nohup hichap GlobalMapping -w ./GM12878 -log GM12878.log -b ~/tools/bowtie2/bowtie2 -i ./GM12878/genome/hg19/hg19
-m PBS -pt 10 4 &
For Hi-C haplotype pipeline, there are two index parameters, maternal and paternal indexes. For example:
$ nohup hichap GlobalMapping -w ./GM12878 -log GM12878.log -b ~/tools/bowtie2/bowtie2 -i ./GM12878/genome/Maternal/Maternal
./GM12878/genome/Paternal/Paternal -m PBS -pt 10 4 &
The key parameter of this command -m(--mode) must be PBS. The parameter -pt (--PBSthreads) 10 4 means that 10 chunks will run mapping tasks parallelly, and each task uses 4 threads. Using the qstat to check the tasks.
$ qstat
Job ID Name User Time Use S Queue
------------------------- ---------------- --------------- -------- - -----
2266086.admin GM12878_R1 hluo 0 R batch
2266087.admin GM12878_R1 hluo 0 R batch
2266088.admin GM12878_R1 hluo 0 R batch
2266089.admin GM12878_R1 hluo 0 R batch
2266090.admin GM12878_R1 hluo 0 R batch
2266091.admin GM12878_R1 hluo 0 R batch
2266092.admin GM12878_R1 hluo 0 R batch
2266093.admin GM12878_R1 hluo 0 R batch
2266094.admin GM12878_R1 hluo 0 R batch
2266095.admin GM12878_R1 hluo 0 R batch
If you are not using the cluster, please use WS API to start mapping tasks.
For traditional Hi-C pipeline, there is only one index parameter. For example:
$ hichap GlobalMapping -w ./GM12878 -log GM12878.log -b ~/tools/bowtie2/bowtie2 -i ./GM12878/genome/hg19/hg19 -m WS -wt 16
For Hi-C haplotype pipeline, there are two index parameters, maternal and paternal indexes. For example:
$ hichap GlobalMapping -w ./GM12878 -log GM12878.log -b ~/tools/bowtie2/bowtie2 -i ./GM12878/genome/Maternal/Maternal
./GM12878/genome/Paternal/Paternal -m WS -wt 16
The key parameter of this command -m(--mode) must be WS. The parameter -wt (--PBSthreads) 16 means that 16 threads will be shared by 4 mapping tasks. That is, 4 chunk mapping tasks are running parallelly and each task occupies 4 threads.
Rescue the unmapped reads. For unmapped reads in GlobalMapping, hichap will search the ligation-junction sites and use the rescuing mode to make full use of sequence information on reads.
For traditional Hi-C pipeline:
$ hichap Rescue -w ./GM12878 -log GM12878.log -e MboI -t 8 -N
For Hi-C haplotype pipeline:
$ hichap Rescue -w ./GM12878 -log GM12878.log -e MboI -t 8
Except for the inputs, the other parameters are same as GlobalMapping ..
Integrate all the mapping information.
For traditional Hi-C pipeline, the fragment parameter(-f) only have one fragment-location file and the SNP parameter should be default (None). For example:
$ hichap bamProcess -w ./GM12878 -log ./GM12878.log -N -f ./GM12878/genome/GATC_hg19_fragments.txt -t 16 --rfo
For Hi-C haplotype pipeline, the fragment parameter(-f) should have two fragment-location files, maternal and paternal files. The SNP parameter should be set. For example:
$ hichap bamProcess -w ./GM12878 -log ./GM12878.log -f ./GM12878/genome/GATC_Maternal_fragments.txt
./GM12878/genome/GATC_Paternal_fragments.txt -s ./GM12878/genome/SNPs/Snps.pickle -t 16 --rfo
The parameter --rfo means filtering the unique reads softly. If your data have high sequencing depths, you can remove this parameter by sacrificing the data utilization.
The filtering sub-command is designed to perform some basic filtering on the mapped Hi-C read pairs:
- Remove redundant PCR duplicates
- Remove the read pair that maps to the same restriction fragment
- assignment maternal interaction pairs
- assignment paternal interaction pairs
- assignment regroup interaction pairs
Here's the command you should type in the terminal:
For traditional Hi-C pipeline
$ hichap filtering -w ./GM12878 -log ./GM12878.log -N -t 16
For Hi-C haplotype pipeline
$ hichap filtering -w ./GM12878 -log ./GM12878.log -t 16
After this sub-command, some bed files will be created under the workspace, such as "Filtered_Bed" folder for traditional Hi-C pipeline, "Allelic_Bed" folder for Hi-C haplotype pipeline. The main file is "**_Valid_sorted.bed", which is the valid Hi-C interactions. This file has 23 columns and you can do some custom processing by using it. The description of each column is :
| ------- | Hi-C interaction |
|---|---|
| column | description |
| 1 | Pair Name |
| 2 | R1 mate Reference |
| 3 | R1 mate Strand |
| 4 | R1 mate Position |
| 5 | R1 mate Length |
| 6 | R1 mate AS score |
| 7 | R1 mate Fragment Middle point |
| 8 | R1 mate SNP Matching num (traditional Hi-C results in 0) |
| 9 | R1 mate Reference |
| 10 | R2 mate Strand |
| 11 | R2 mate Position |
| 12 | R2 mate Length |
| 13 | R2 mate AS score |
| 14 | R2 mate Fragment Middle point |
| 15 | R2 mate SNP Matching num (traditional Hi-C results in 0) |
| -------- | candidate mate if it is possible |
| 16 | Candidate mate Reference |
| 17 | Candidate mate Strand |
| 18 | Candidate mate Position |
| 19 | Candidate mate Length |
| 20 | Candidate mate AS score |
| 21 | Candidate mate Fragment Middle point |
| 22 | Candidate mate SNP Matching num (traditional Hi-C results in 0) |
| 23 | Candidate Index for which mate. |
For Hi-C haplotype pipeline, the output files of haplotype interactions have the targets like "M_M", "P_P", "M_P", "P_M", "Bi_Allelic". The "M_M" represents the maternal-maternal interactions. The "M_P" represents the maternal-paternal interactions. "Bi_Allelic" represents no allelic assignment. The files have 5 columns. The description of each column is:
| ----- | Haplotype-resolved Hi-C interactions |
|---|---|
| column | description |
| 1 | chromosome ID for interaction loci 1 |
| 2 | fragment ID for interaction loci 1 |
| 3 | chromosome ID for interaction loci 2 |
| 4 | fragment ID for interaction loci 2 |
| 5 | assignment target (R1 means assigned by R1, R2 means assigned by R2, Both means both mate can be assigned) |
Interaction Matrix Construction. The cooler format file will be generated. For this sub-command, You need to set the output-folder path. The matrix will be saved in cooler files at different resolutions
For traditional Hi-C pipeline
$ hichap matrix -b GM12878_R1_workspace/Filtered_Bed GM12878_R2_worspace/Filtered_Bed -N -o ./GM12878_Matrix
-gs ./genome/genomeSize -wR 2000000 1000000 -lR 200000 40000 20000
For Hi-C haplotype pipeline
$ hichap matrix -b GM12878_R1_workspace/Allelic_Bed GM12878_R2_workspace/Allelic_Bed -o ./GM12878_Matrix
-gs ./genome/genomeSize -wR 5000000 2000000 -lR 500000 40000
The Imputation parameters can be changed. try --help for more information.
!!! Notice !!!
The traditional matrix in cool are balanced by ICE.
The haplotype-resolved matrices are not stored as balance data in cool way. The raw count values in haplotype-resolved matrices are already corrected by HiCHap and the type is float.
The Gap file are saved into NPZ file.U can use numpy to load it.
Loading the Matrix in cooler. Open a python interpreter and follow the code below:
>>> import cooler
>>> GM12878_T = cooler.Cooler('Merged_Traditional_Multi.cool::40000')
>>> GM12878_T.matrix(balance = False).fetch('1') #Get the raw chromosome 1 Matrix
>>> GM12878_T.matrix(balance = True).fetch('1') #Get the balanced chromosome 1 Matrix
>>> # Haplotype-resolved Matrix
>>> GM12878_Haplotype = cooler.Cooler('Merged_Imputated_Haplotype_Multi.cool::40000')
>>> GM12878_Haplotype.matrix(balance = False).fetch('M1') #Get chromosome 1 Maternal Matrix
>>> GM12878_Haplotype.matrix(balance = False).fetch('P1') #Get chromosome 1 Paternal Matrix
More infomation about cooler format here
Structure analysis is integrated in the module StructureFind. The source code can be found in the lib/StructureFind.py.
Use the API like :
>>> from HiCHap.StructureFind import StructureFind
>>> #============= Compartment==============
>>> ## For traditional Hi-C
>>> GM_T_PC = StructureFind(cooler_fil = 'Merged_Traditional_Multi.cool', Res = 500000, Allelic = False)
>>> GM_T_PC.run_Compartment(OutPath = 'Traditonal_PC', plot = True, MS = 'IF', SA = False)
>>> ## For haplotype-resolved Hi-C
>>> GM_M_PC = StructureFind(cooler_fil = 'Merged_Imputated_Haplotype_Multi.cool', Res = 500000, Allelic = 'Maternal')
>>> GM_M_PC.run_Compartment(OutPath = 'Maternal_PC', plot = True, MS = 'IF', SA = False,
Tranditional_PC_file = 'Traditional_PC/Traditional_PC_Compartment_500K.txt')
>>> GM_P_PC = StructureFind(cooler_fil = 'Merged_Imputated_Haplotype_Multi.cool', Res = 500000, Allelic = 'Paternal')
>>> GM_P_PC.run_Compartment(OutPath = 'Paternal_PC', plot = True, MS = 'IF', SA = False,
Tranditional_PC_file = 'Traditional_PC/Traditional_PC_Compartment_500K.txt')
>>> #============= TADs calling=============
>>> ## For traditional Hi-C
>>> GM_tads_T = StructureFind(cooler_fil = 'Merged_Traditional_Multi.cool', Res = 40000, Allelic = False)
>>> GM_tads_T.run_TADs(OutPath = 'Traditional_TADs', plot = True)
>>>
>>> ## For haplotype-resolved Hi-C
>>> GM_tads_M = StructureFind(cooler_fil = 'Merged_Imputated_Haplotype_Multi.cool', Res = 40000, Allelic = 'Maternal')
>>> GM_tads_M.run_TADs(OutPath = 'Maternal_TADs', plot = True)
>>> GM_tads_P = StructureFind(cooler_fil = 'Merged_Imputated_Haplotype_Multi.cool', Res = 40000, Allelic = 'Paternal')
>>> GM_tads_P.run_TADs(OutPath = 'Paternal_TADs', plot = True)
>>> #============= Loops calling=============
>>> ## For traditonal Hi-C
>>> GM_Loop_T = StructureFind(cooler_fil = 'Merged_Traditional_Multi.cool', Res = 40000, Allelic = False)
>>> GM_Loop_T.run_Loops(OutPath = 'Traditional_Loops', plot = True)
>>> ## For haplotype-resolved Hi-C
>>> GM_Loop_M = StructureFind(cooler_fil = 'Merged_Imputated_Haplotype_Multi.cool', Res = 40000, Allelic = 'Maternal')
>>> GM_Loop_M.run_Loops(OutPath = 'Maternal_Loops', plot = True)
>>> GM_Loop_P = StructureFind(cooler_fil = 'Merged_Imputated_Haplotype_Multi.cool', Res = 40000, Allelic = 'Paternal')
>>> GM_Loop_P.run_Loops(OutPath = 'Paternal_Loops', plot = True)
Notice that the most important parameter is Allelic, False for traditional Hi-C and Maternal/Paternal for Maternal/paternal..
You can read the source code in StuctureFind.py for more details.
The calculation method of Allelic Specificity is integrated in the AllelicSpecificity module.
Using the maternal and paternal PC1 values as input. with the format as:
- chromosome ID. 2) PC1 values
Each row indicates a chromatin bin and its pc values. Bins are ordered from 5' to 3'.
$ less GM12878_Maternal_PC.txt
1 0.007763622511004936
1 0.03661900228230602
1 0.031072969611405883
1 0.03303907288771536
1 0.03185222709883546
...
Use the API like:
>>> from HiCHap.AllelicSpecificity import CompartmentAllelicSpecificity
>>> Allel_PC = CompartmentAllelicSpecificity(Maternal_PC = 'GM12878_Maternal_PC.txt',
Paternal_PC = 'GM12878_Paternal_PC.txt',
Res = 500000)
>>> Allel_PC.Running('Output.txt')
The output file is similar to the input file. with the format as:
- chromosome ID
- position
- Maternal PC value
- Paternal PC value
- difference
- p-value
- q-value
Using the Candidate boundaries as input. TXT file contains 3 columns format as
- chromosome ID.
- Maternal Boundary
- Paternal Boundary
Each row indicates a pair of candidate boundary to calculate the allel-specificity. Maternal and Paternal boundary can be different (Same boundary with a little translation.) We suggest the distance of translation should less than 3 bins.
$ less Candidate_Boundary.txt
1 800000 800000
1 1240000 1240000
1 1680000 1680000
1 1840000 1840000
1 2080000 2040000
1 2320000 2320000
...
Use the API like:
>>> from HiCHap.AllelicSpecificity import BoundaryAllelicSpecificity
>>> Allel_Boundary = BoundaryAllelicSpecificity(cooler_fil = 'Merged_Imputated_Haplotype_Multi.cool',
Boundary_fil = 'Candidate_Boundary.txt',
Res = 40000, offset = 10)
>>> Allel_Boundary.Running('OutPut.txt')
The output file is similar to the input file. with the format as:
- chromosome ID
- Maternal boundary position
- Paternal boundary position
- Mean value of Maternal boundary
- Mean value of Paternal boundary
- stat
- p-value
- q-value
Using the Candidate loops as input. TXT file contains 5 columns format as
- chromosome ID.
- Maternal loop loci 1.
- Maternal loop loci 2.
- Paternal loop loci 1.
- Paternal loop loci 2
like:
$ less Candidate_Loop.txt
1 2320000 2560000 2320000 2560000
1 13840000 14160000 13840000 14160000
1 35320000 35640000 35320000 35640000
1 47640000 48160000 47640000 48160000
...
Use the API like:
>>> from HiCHap.AllelicSpecificity import LoopAllelicSpecificity
>>> Allel_Loop = LoopAllelicSpecificity(cooler_uri = 'Merged_Imputated_Haplotype_Multi.cool',
Loop_file = 'Candidate_Loop.txt',
Res = 40000)
>>> Allel_Loop.Running('OutPut.txt')
The output file is similar to the input file. with the format as:
- chromosome ID
- Maternal loop loci 1
- Maternal loop loci 2
- Paternal loop loci 1
- Paternal loop loci 2
- Maternal loop Contact strength
- Paternal loop Contact strength
- quantile ratio
- log2(fold-change)
- stat
- p-value
Luo, H., Li, X., Fu, H. et al. HiCHap: a package to correct and analyze the diploid Hi-C data. BMC Genomics 21, 746 (2020). https://doi.org/10.1186/s12864-020-07165-x