| Issues | Pull Requests | Repository Size | Languages | License | Tests |
|---|---|---|---|---|---|
  |
  |
  |
  |
 |
This readme is a work in progress!
What is the R-ODAF?
It is an analysis framework geared toward the use of 'omics data in toxicology experiments. It aims to use commonly available open-source tools for analysis of transcriptomic data, specifically using DESeq2 for the determination of differentially expressed genes (DEGs).
This R-ODAF repository, which was forked from the main branch, is under development by researchers at Health Canada and has been used primarily in the context of regulatory toxicology. The intended use is for processing high-throughput sequencing data from FASTQ files to DEG lists, and to generate exploratory analyses for use in toxicology research.
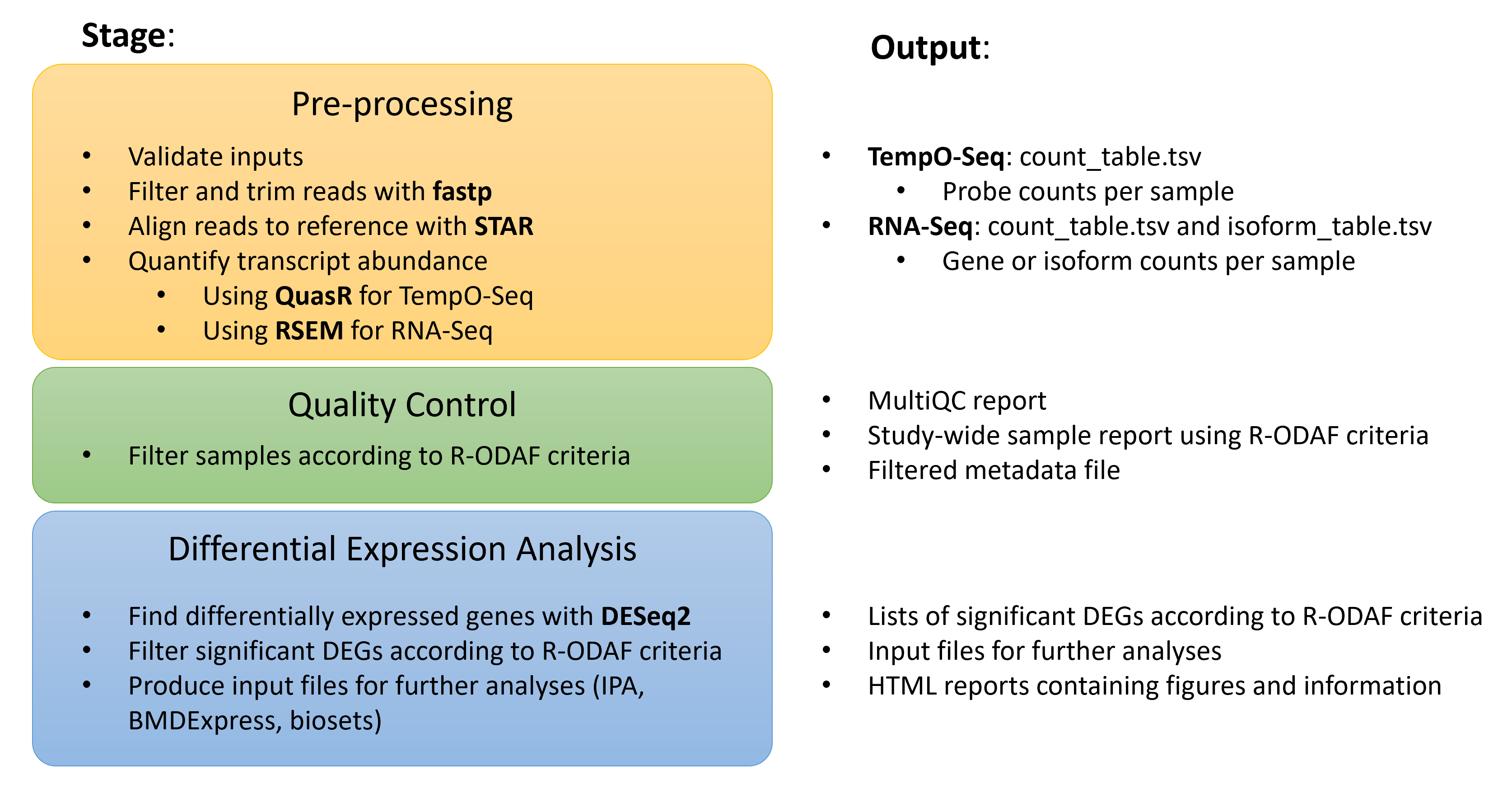
This repository can be used to analyze transcriptomic datasets generated by either TempO-seq or RNA-seq. It generates:
- study-wide HTML reports on the quality of HTS data using MultiQC
- lists of differentially expressed genes (DEGS), filtered according to R-ODAF filtering criteria as set out in the publication
- output files formatted for use in BMD modeling or IPA analysis
- exploratory data visualization reports for DEGs, inspired by the regionReport DESeq2 reports
- interactive tables and plots related to analysis of gene expression
This repository provides no warranty or guarantees on the quality of your analysis. It is used actively in research projects and is not intended to provide any type of authoritative assessment of your data.
There may be other tools that are more applicable to your goals. Indeed, if you are not undertaking toxicogenomics experiments, then that is likely the case.
In its current state of development, this repository should be used by individuals (or teams) who are experienced with running bioinformatic analysis in a linux environment. Proficiency in R is also very helpful. It is necessary to understand scripting and how to process data. We encourage users to learn if they are interested in pursuing this type of data analysis; to that end, we provide a users guide below detailing the steps required to process data, and best practices for using this analysis pipeline.
For reasons of reproducibility, this respository uses the following annotation versions:
org.Hs.eg.db 3.14.0 (https://bioconductor.org/packages/release/data/annotation/html/org.Hs.eg.db.html)
org.Mm.eg.db 3.14.0 (https://bioconductor.org/packages/release/data/annotation/html/org.Mm.eg.db.html)
You should start by cloning the repository locally, to a computer that can handle analysis of your data and where your raw data is locally accessible.
You can clone the repository using this command:
git clone https://github.com/R-ODAF/R-ODAF_Health_Canada.git
Typically, when undertaking a new analysis, it is a good idea to have a separate folder for each project. This makes it easier to manage, and also makes your code more portable. Working in RStudio facilitates these tasks.
Therefore, it is a good idea to open the cloned repository in RStudio as a project (there is already a project file by default in this repository, so RStudio should recognize it). This tells RStudio where to look for files, which is better than setting a working directory.
Note that R Studio Server has a built-in terminal in which unix commands can be run. However, you should use the command line in a separate terminal rather than using the one in R Studio Server. This is because Rstudio will try to use packages installed in user directories rather than those installed in conda experiments, causing the pipeline run to abort.
- conda
- snakemake > 6.0 (see note on versions below)
- R
We recommend installing snakemake within its own conda environment to avoid conflicts.
NOTE on Snakemake versions : Snakemake v8.0 and above introduced changes to the snakemake command used to run the workflow. The commands given in this README apply to v8.0 and above. If you are using an earlier version of Snakemake, replace --software-deployment-method conda with --use-conda
For more information: https://snakemake.readthedocs.io/en/stable/project_info/history.html#detailed-breaking-changes
If you do not yet have mamba, install it.
conda install -n base -c conda-forge mamba
Follow the installation steps provided in the snakemake documentation https://snakemake.readthedocs.io/en/stable/getting_started/installation.html#full-installation
conda activate base
mamba create -c conda-forge -c bioconda -n snakemake snakemake
To activate this environment:
conda activate snakemake
Other dependencies are managed by snakemake via the creation of conda environments. The conda environments needed for various steps of the workflow are defined in a series of .yml files in workflow/envs. These .yml files are human-readable if you're curious.
Environments must be installed prior to running the workflow because of additional dependencies that must be added to the R-ODAF_reports environment via the install.R script included in this repo. If you use snakemake to run the workflow without first installing and fixing the environments, the workflow will not run to completion.
NOTE on Snakemake versions : Snakemake v8.0 and above introduced changes to the snakemake command used to run the workflow. The commands given in this README apply to v8.0 and above. If you are using an earlier version of Snakemake, replace --software-deployment-method conda with --use-conda
From inside the repo top-level directory, run:
snakemake --cores 32 --software-deployment-method conda --conda-create-envs-only
Choose the number of cores according to what is available in your system.
Then install additional software in the reports environment:
conda run -p $(grep -rl "R-ODAF_reports" .snakemake/conda/*.yaml | sed s/\.yaml//) Rscript install.R
If you intend to run this analysis on multiple datasets, it can be advantageous to instead install the environments in a persistent location, and point to this location with snakemake's --conda-prefix flag. This avoids having to install the environments multiple times, which saves both time and disk place.
For example, the code below installs the environments in ~/snakemake_envs. Using this method, every time the analysis is run with snakemake, the command must include --conda-prefix ~/snakemake_envs
mkdir ~/snakemake_envs #Place and name this directory as you wish, and use correctly later with --conda-prefix
snakemake --cores 32 --software-deployment-method conda --conda-create-envs-only --conda-prefix ~/snakemake_envs/
conda run -p $(grep -rl "R-ODAF_reports" ~/snakemake_envs/*.yaml | sed s/\.yaml//) Rscript install.R
When running the workflow, remember to use the --conda-prefix flag.
More details as well as template files can be found in the inputs subdirectories.
- Metadata file (inputs/metadata/metadata.txt)
- Contrasts file (inputs/contrasts/contrasts.txt)
- Config file (inputs/config/config.yaml)
- Reference files (inputs/reference/{reference_genome}.fa, inputs/reference/{reference_genome}.gtf) - see "Reference genome files" below
- Note that files may be stored elsewhere and their location given in the config parameter "genomedir"
- TempO-seq experiments only : TempO-Seq manifest file matching the genome reference files. We have created standardized TempO-Seq manifests, available at https://github.com/EHSRB-BSRSE-Bioinformatics/unify_temposeq_manifests/
- Note that files may be stored elsewhere and their location given in the config parameter "biospyder_dbs"
- The manifest file name must be provided in the config parameter "biospyder_manifest_file"
- Raw fastq files (inputs/raw/) - see "Sample names" below
You must provide a reference sequence for alignment of the raw reads. For TempO-Seq experiments, these are provided by BioSpyder; for RNA-seq experiments they can be obtained through your favorite database. This is currently beyond the scope of this guide. Ensure you have some type of annotation file (GTF format) available as well, to dictate which sequences in the FASTA file correspond to which genes or probes. These will be used to create a STAR index within the directory where you store your reference genome in FASTA format.
A column labeled sample_ID must be included in the metadata table, containing a unique identifier for each sample. This identifier must match the fastq.gz file names in the following way:
For single-end sequencing: {sample_ID}.fastq.gz
For paired-end sequencing: {sample_ID}.R1.fastq.gz and {sample_ID}.R2.fastq.gz
section coming soon
NOTE on Snakemake versions : Snakemake v8.0 and above introduced changes to the snakemake command used to run the workflow. The commands given in this README apply to v8.0 and above. If you are using an earlier version of Snakemake, replace --software-deployment-method conda with --use-conda
We strongly recommend using snakemake's dry run functionality to check for errors before beginning an actual run. (See https://snakemake.readthedocs.io/en/v7.32.2/executing/cli.html).
Use the flags --dry-run, or -n
For example,
snakemake -s ./workflow/Snakefile --cores 32 --software-deployment-method conda -n
To run the whole pipeline:
snakemake -s ./workflow/Snakefile --software-deployment-method conda --cores 32
Choose the number of cores according to what is available in your system.
This workflow is modularized, so you can run (or re-run) some sections independently if needed. This can be beneficial if, for example:
- you choose to manually remove samples (by providing a list in file "remove.txt" in inputs/metadata) and want to re-run the QC and differential expression steps.
- you want to try differential expression analysis with different parameters
To run only the preprocessing steps and get a count table for TempO-Seq datasets:
snakemake -s ./workflow/modules/4-preprocess_temposeq.smk --software-deployment-method conda --cores 32
To run preprocessing steps and get a count table for RNA-Seq datasets:
snakemake -s ./workflow/modules/4-preprocess_rnaseq.smk --software-deployment-method conda --cores 32
Once the preprocessing steps are complete, you can run (or re-run) the quality control steps. These apply the R-ODAF filters on all samples and produce a multiQC report, a study-wide report on quality control filters according to R-ODAF criteria, and a filtered metadata file.
snakemake -s ./workflow/modules/5-qc.smk --software-deployment-method conda --cores 32
Once the preprocessing and quality control steps are complete, you can run (or re-run) differential analysis via DESeq2 and production of DEG reports. This will produce DEG lists, exploratory reports, and interactive gene expression reports that incorporate DEG filtering according to R-ODAF criteria.
snakemake -s ./workflow/modules/6-diffexp.smk --software-deployment-method conda --cores 32
Note that you will have to delete dummy output file "reports_complete" from your top-level directory to re-run this step
Each analysis folder contains three subdirectories: DEG_reports, DEG_lists, and RData.
DEG_reports contains 3 html reports:
- RNA-Seq report has volcano plots for each contrast, PCA plots to see clustering of groups (note the PCAs are identical across all comparisons, as they are not based on DEGs), DEG heatmaps, and plots of log2foldchange and normalized counts for the best 20 features (DEGs)
- data_explorer report has a table of significant DEGs and normalized count plots for the top 1000 (by absolute fold change) genes.
- stats report has information about the sample metadata, which contrasts were used in DESeq2 and how many DEGs were found per contrast, number of samples per group and a series of descriptive plots
DEG_lists contains lists of differentially expressed genes, as well as DEG information formatted to be used as input for further analysis tools (ex. IPA).
- *_Per_sample_CMP.txt - A matrix of copies per million reads of each gene for each sample.
- *_Per_sample_normalized_counts.txt - a matrix of counts per gene per sample, normalized by [???]
- *_DESeq_output_ALL.txt - DESeq2 results table for all features
- *_DESeq_output_all_genes.txt - DESeq2 results table for all genes (a filtered subset from *_DESeq_output_ALL.txt
- *_DESeq_output_significant.txt - DESeq2 results table for features that passed all filters laid out in the R-ODAF framework (see "Methods Summary" in the RNA-Seq report for details)
- *_DEG_summary.txt - a table of DEGs found per contrast
RData contains an .RData file that can be used to re-import results to R for further analysis if needed. It also contains records of the contrasts and config file used.
I'm not surprised if you have questions. This is very much a draft of the workflow, and is incomplete except for serving highly experienced users. Please reach out if you have questions or issues, and feel free to submit an issue or PR with any suggestions you might have.