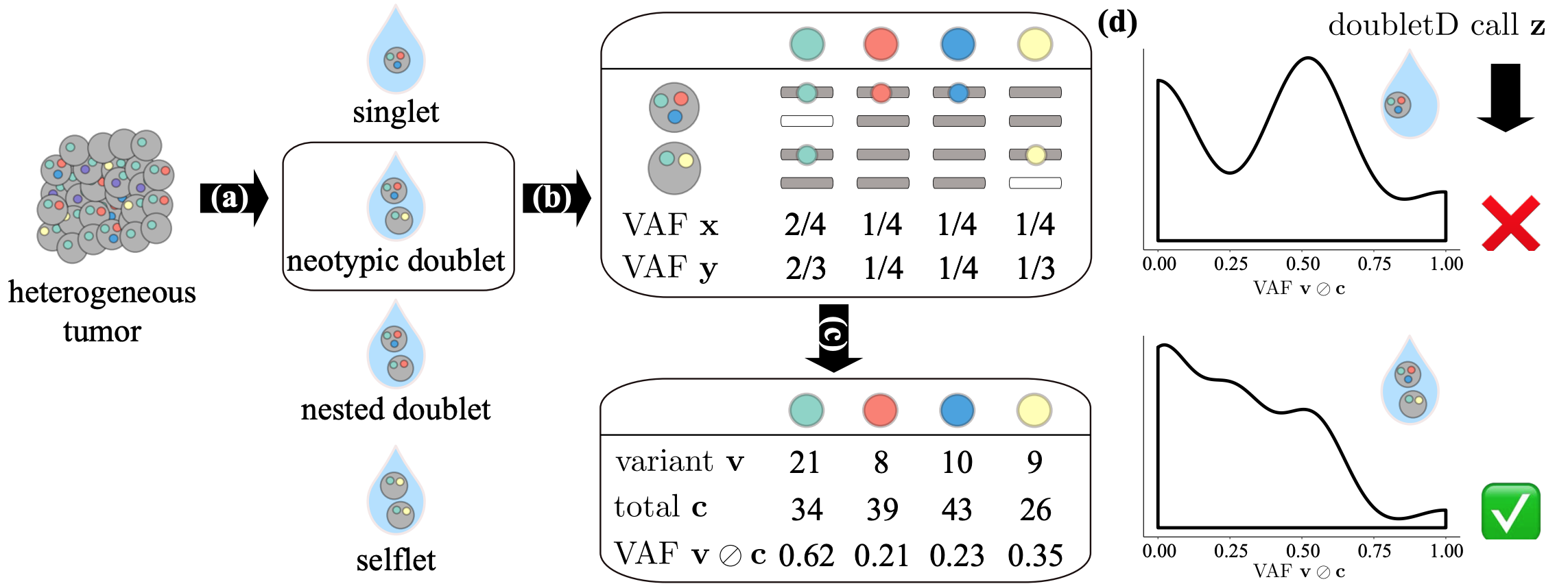
 (a) The first step of most single-cell sequencing technologies involves cell capture where the goal is to encapsulate single cells into droplets, known as singlets.
However, errors in this process can lead to three kind of doublets -- neotypic doublets, nested doublets and selflets.
(b) The cells in each isolated droplet i undergo whole-genome amplification and sequencing independently.
These processes introduce errors such as allelic dropouts and imbalance in amplification.
(c) The resulting aligned reads are used for variant calling yielding alternate v_{i,j} and total c_{i,j} read counts at each locus of interest j.
(d) doubletD uses the observed variant allele frequencies v_{i,j}/c_{i,j} as the key signal, while accounting for sequencing and amplification errors to detect doublets in the sample.
(a) The first step of most single-cell sequencing technologies involves cell capture where the goal is to encapsulate single cells into droplets, known as singlets.
However, errors in this process can lead to three kind of doublets -- neotypic doublets, nested doublets and selflets.
(b) The cells in each isolated droplet i undergo whole-genome amplification and sequencing independently.
These processes introduce errors such as allelic dropouts and imbalance in amplification.
(c) The resulting aligned reads are used for variant calling yielding alternate v_{i,j} and total c_{i,j} read counts at each locus of interest j.
(d) doubletD uses the observed variant allele frequencies v_{i,j}/c_{i,j} as the key signal, while accounting for sequencing and amplification errors to detect doublets in the sample.
- Installation
- Using conda(recommended)
- Using pip (alternative)
- Usage instructions
$ conda install -c bioconda doubletd-
Clone the repository
$ git clone https://github.com/elkebir-group/doubletD.git
-
Install doubletD using pip
$ cd doubletD ` $ pip install ./
The input for doubletD is a text based with two input comma-separated dataframes -- one containing the total read counts and another containing the alternate read counts.
For both the files, each row is a different droplet and each column is a loci.
See data/sample_DP.tsv and data/sample_AD.tsv for an example for both files.
The output is also a dataframe with each row for a different droplet and columns, from left to right, posterior probability that the droplet is a singlet, posterior probability that the droplet is a doublet and prediction for the droplet to be either 'singlet' or 'doublet'.
See data/sample_prediction.tsv for an example.
Parameters with default value None are estimated from data
usage: doubletd [-h] [--inputTotal INPUTTOTAL]
[--inputAlternate INPUTALTERNATE] [--delta DELTA]
[--beta BETA] [--mu_hetero MU_HETERO] [--mu_hom MU_HOM]
[--alpha_fp ALPHA_FP] [--alpha_fn ALPHA_FN] [-o OUTPUTFILE]
[--noverbose] [--binomial] [--prec PREC] [--missing]
optional arguments:
-h, --help show this help message and exit
--inputTotal INPUTTOTAL
csv file with a table of total read counts for each
position in each cell
--inputAlternate INPUTALTERNATE
csv file with a table of alternate read counts for
each position in each cell
--delta DELTA expected doublet rate [0.1]
--beta BETA allelic dropout (ADO) rate [0.05]
--mu_hetero MU_HETERO
heterozygous mutation rate [None]
--mu_hom MU_HOM homozygous mutation rate [None]
--alpha_fp ALPHA_FP copy false positive error rate [None]
--alpha_fn ALPHA_FN copy false negative error rate [None]
-o OUTPUTFILE, --outputfile OUTPUTFILE
output file name
--noverbose do not output statements from internal solvers
[default is false]
--binomial use binomial distribution for read count model
[default is false]
--prec PREC precision for beta-binomial distribution [None]
--missing use missing data in the model? [No]
Here we will show an example of how to run doubletD.
The input files are located in the example directory.
We run doubletD with a prior doublet probabiltity of 0.2 and ADO rate of 0.05 without using missing data in our model.
$ doubletd --inputAlternate example/AD.csv --inputTotal example/DP.csv --delta 0.2 --beta 0.05 -o example/prediction.tsv
This command generates output file prediction.tsv in directory example.
