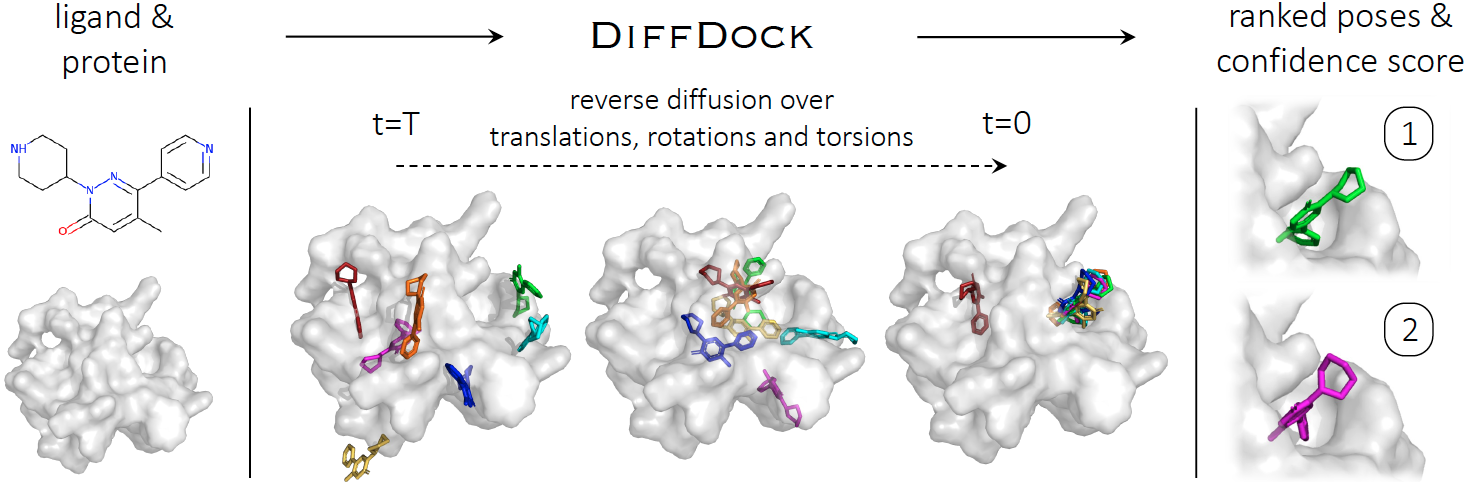
Implementation of DiffDock, state-of-the-art method for molecular docking, by Gabriele Corso*, Hannes Stark*, Bowen Jing*, Regina Barzilay and Tommi Jaakkola. This repository contains all code, instructions and model weights necessary to run the method or to retrain a model. If you have any question, feel free to open an issue or reach out to us: gcorso@mit.edu, hstark@mit.edu, bjing@mit.edu.
The repository also contains all the scripts to run the baselines and generate the figures.
Additionally, there are visualization videos in visualizations.
You might also be interested in this awesome interactive online tool by Simon Duerr on Hugging Face for running DiffDock and visualising the predicted structures on your browser. There is also a Google Colab notebook to run DiffDock by Brian Naughton.
The files in data contain the names for the time-based data split.
If you want to train one of our models with the data then:
- download it from zenodo
- unzip the directory and place it into
datasuch that you have the pathdata/PDBBind_processed
We will set up the environment using Anaconda. Clone the current repo
git clone https://github.com/gcorso/DiffDock.git
This is an example for how to set up a working conda environment to run the code (but make sure to use the correct pytorch, pytorch-geometric, cuda versions or cpu only versions):
conda create --name diffdock python=3.8
conda activate diffdock
conda install pytorch pytorch-cuda=11.7 -c pytorch -c nvidia
pip install torch-scatter torch-sparse torch-cluster torch-spline-conv torch-geometric -f https://data.pyg.org/whl/torch-1.13.0+cu117.html
python -m pip install PyYAML scipy "networkx[default]" biopython rdkit-pypi e3nn spyrmsd pandas biopandas
Then you need to install ESM that we use both for protein sequence embeddings and for the protein structure prediction in case you only have the sequence of your target. Note that OpenFold (and so ESMFold) requires a GPU. If you don't have a GPU, you can still use DiffDock with existing protein structures.
pip install "fair-esm[esmfold]"
pip install 'dllogger @ git+https://github.com/NVIDIA/dllogger.git'
pip install 'openfold @ git+https://github.com/aqlaboratory/openfold.git@4b41059694619831a7db195b7e0988fc4ff3a307'
We support multiple input formats depending on whether you only want to make predictions for a single complex or for many at once.
The protein inputs need to be .pdb files or sequences that will be folded with ESMFold. The ligand input can either be a SMILES string or a filetype that RDKit can read like .sdf or .mol2.
For a single complex: specify the protein with --protein_path protein.pdb or --protein_sequence GIQSYCTPPYSVLQDPPQPVV and the ligand with --ligand ligand.sdf or --ligand "COc(cc1)ccc1C#N"
For many complexes: create a csv file with paths to proteins and ligand files or SMILES. It contains as columns complex_name (name used to save predictions, can be left empty), protein_path (path to .pdb file, if empty uses sequence), ligand_description (SMILE or file path) and protein_sequence (to fold with ESMFold in case the protein_path is empty).
An example .csv is at data/protein_ligand_example_csv.csv and you would use it with --protein_ligand_csv protein_ligand_example_csv.csv.
And you are ready to run inference:
python -m inference --protein_ligand_csv data/protein_ligand_example_csv.csv --out_dir results/user_predictions_small --inference_steps 20 --samples_per_complex 40 --batch_size 10 --actual_steps 18 --no_final_step_noise
When providing the .pdb files you can run DiffDock also on CPU, however, if possible, we recommend using a GPU as the model runs significantly faster. Note that the first time you run DiffDock on a device the program will precompute and store in cache look-up tables for SO(2) and SO(3) distributions (typically takes a couple of minutes), this won't be repeated in following runs.
Download the data and place it as described in the "Dataset" section above.
First run:
python datasets/pdbbind_lm_embedding_preparation.py
Use the generated file data/pdbbind_sequences.fasta to generate the ESM2 language model embeddings using the library https://github.com/facebookresearch/esm by installing their repository and executing the following in their repository:
python scripts/extract.py esm2_t33_650M_UR50D pdbbind_sequences.fasta embeddings_output --repr_layers 33 --include per_tok --truncation_seq_length 4096
This generates the embeddings_output directory which you have to copy into the data folder of our repository to have data/embeddings_output.
Then run the command:
python datasets/esm_embeddings_to_pt.py
We first generate the language model embeddings for the testset, then run inference with DiffDock, and then evaluate the files that DiffDock produced:
python datasets/esm_embedding_preparation.py --protein_ligand_csv data/testset_csv.csv --out_file data/prepared_for_esm_testset.fasta
git clone https://github.com/facebookresearch/esm
cd esm
pip install -e .
cd ..
HOME=esm/model_weights python esm/scripts/extract.py esm2_t33_650M_UR50D data/prepared_for_esm_testset.fasta data/esm2_output --repr_layers 33 --include per_tok
python -m inference --protein_ligand_csv data/testset_csv.csv --out_dir results/user_predictions_testset --inference_steps 20 --samples_per_complex 40 --batch_size 10 --actual_steps 18 --no_final_step_noise
python evaluate_files.py --results_path results/user_predictions_testset --file_to_exclude rank1.sdf --num_predictions 40
Train the large score model:
python -m train --run_name big_score_model --test_sigma_intervals --esm_embeddings_path data/esm2_3billion_embeddings.pt --log_dir workdir --lr 1e-3 --tr_sigma_min 0.1 --tr_sigma_max 19 --rot_sigma_min 0.03 --rot_sigma_max 1.55 --batch_size 16 --ns 48 --nv 10 --num_conv_layers 6 --dynamic_max_cross --scheduler plateau --scale_by_sigma --dropout 0.1 --remove_hs --c_alpha_max_neighbors 24 --receptor_radius 15 --num_dataloader_workers 1 --cudnn_benchmark --val_inference_freq 5 --num_inference_complexes 500 --use_ema --distance_embed_dim 64 --cross_distance_embed_dim 64 --sigma_embed_dim 64 --scheduler_patience 30 --n_epochs 850
The model weights are saved in the workdir directory.
Train a small score model with higher maximum translation sigma that will be used to generate the samples for training the confidence model:
python -m train --run_name small_score_model --test_sigma_intervals --esm_embeddings_path data/esm2_3billion_embeddings.pt --log_dir workdir --lr 1e-3 --tr_sigma_min 0.1 --tr_sigma_max 34 --rot_sigma_min 0.03 --rot_sigma_max 1.55 --batch_size 16 --ns 24 --nv 6 --num_conv_layers 5 --dynamic_max_cross --scheduler plateau --scale_by_sigma --dropout 0.1 --remove_hs --c_alpha_max_neighbors 24 --receptor_radius 15 --num_dataloader_workers 1 --cudnn_benchmark --val_inference_freq 5 --num_inference_complexes 500 --use_ema --scheduler_patience 30 --n_epochs 300
In practice, you could also likely achieve the same or better results by using the first score model for creating the samples to train the confidence model, but this is what we did in the paper.
The score model used to generate the samples to train the confidence model does not have to be the same as the score model that is used with that confidence model during inference.
Train the confidence model by running the following:
python -m confidence.confidence_train --original_model_dir workdir/small_score_model --run_name confidence_model --inference_steps 20 --samples_per_complex 7 --batch_size 16 --n_epochs 100 --lr 3e-4 --scheduler_patience 50 --ns 24 --nv 6 --num_conv_layers 5 --dynamic_max_cross --scale_by_sigma --dropout 0.1 --all_atoms --remove_hs --c_alpha_max_neighbors 24 --receptor_radius 15 --esm_embeddings_path data/esm2_3billion_embeddings.pt --main_metric loss --main_metric_goal min --best_model_save_frequency 5 --rmsd_classification_cutoff 2 --cache_creation_id 1 --cache_ids_to_combine 1 2 3 4
first with --cache_creation_id 1 then --cache_creation_id 2 etc. up to 4
Now everything is trained and you can run inference with:
python -m evaluate --model_dir workdir/big_score_model --ckpt best_ema_inference_epoch_model.pt --confidence_ckpt best_model_epoch75.pt --confidence_model_dir workdir/confidence_model --run_name DiffDockInference --inference_steps 20 --split_path data/splits/timesplit_test --samples_per_complex 40 --batch_size 10 --actual_steps 18 --no_final_step_noise
@article{corso2023diffdock,
title={DiffDock: Diffusion Steps, Twists, and Turns for Molecular Docking},
author = {Corso, Gabriele and Stärk, Hannes and Jing, Bowen and Barzilay, Regina and Jaakkola, Tommi},
journal={International Conference on Learning Representations (ICLR)},
year={2023}
}
MIT
We thank Wei Lu and Rachel Wu for pointing out some issues with the code.