[bioRxiv], [Uni-Fold Colab], [Hermite™]
We proudly present Uni-Fold as a thoroughly open-source platform for developing protein models beyond AlphaFold. Uni-Fold introduces the following features:
- Reimplemented AlphaFold and AlphaFold-Multimer models in PyTorch framework. This is currently the first (if any else) open-source repository that supports training AlphaFold-Multimer.
- Model correctness proved by successful from-scratch training with equivalent accuracy, both monomer and multimer included.
- Highest efficiency among existing AlphaFold implementations (to our knowledge).
- Easy distributed training based on Uni-Core, as well as other conveniences including half-precision training (
float16/bfloat16), per-sample gradient clipping, and fused CUDA kernels.
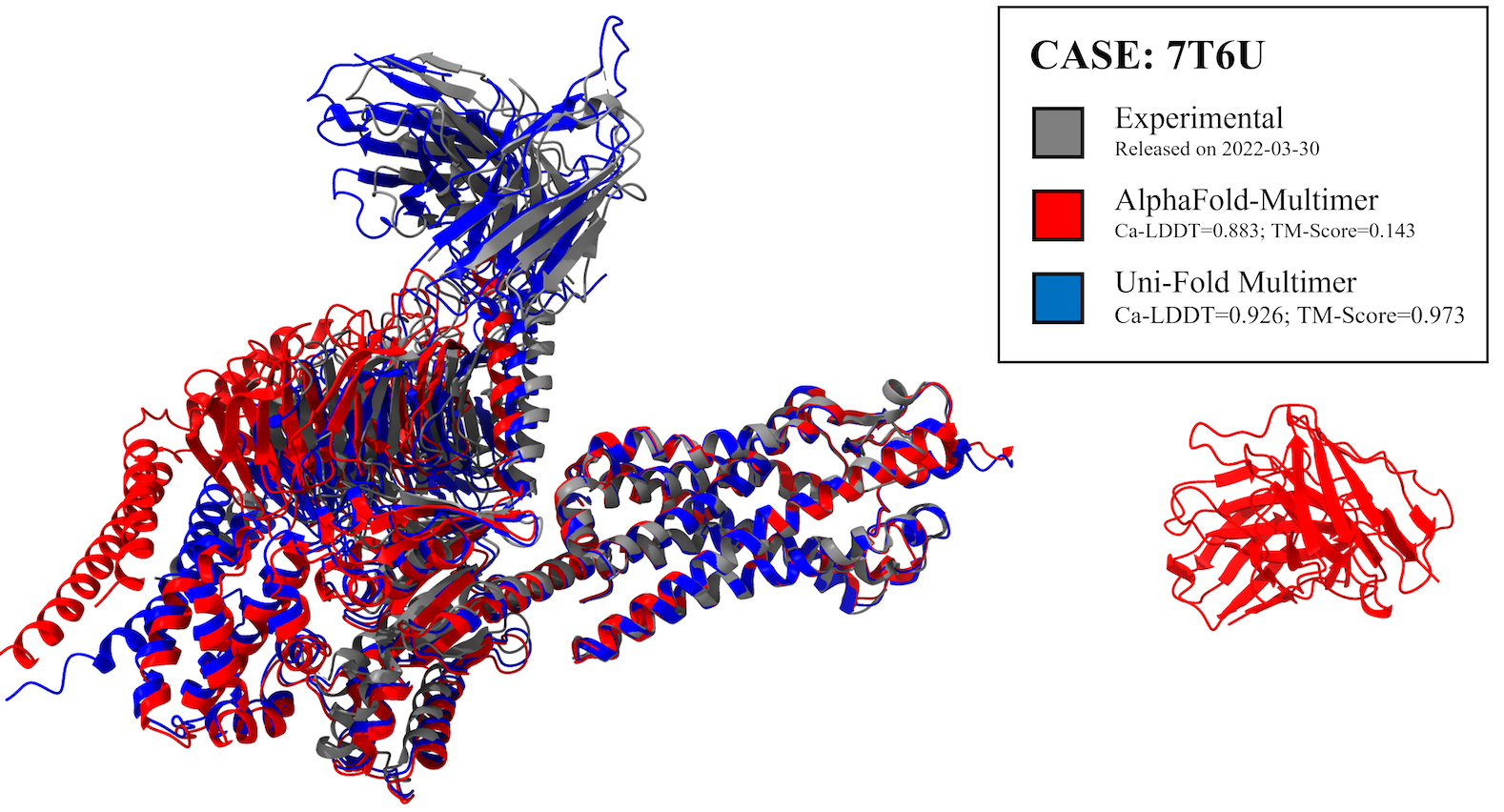
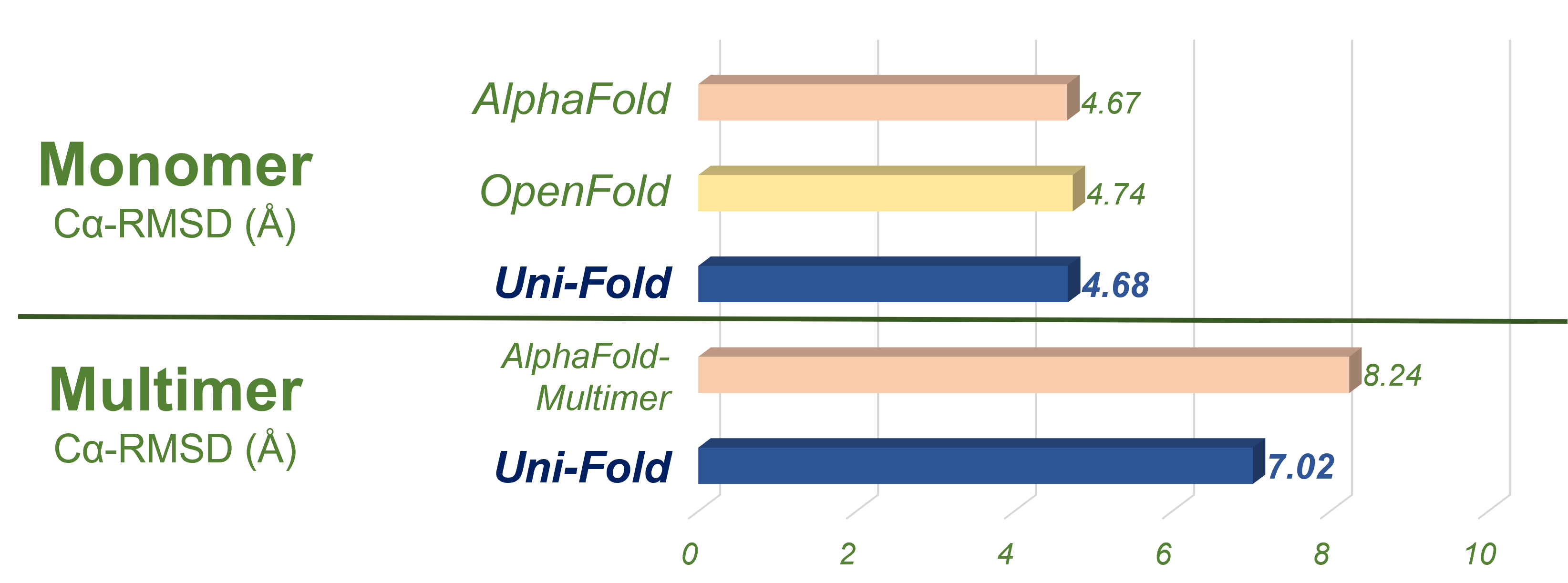
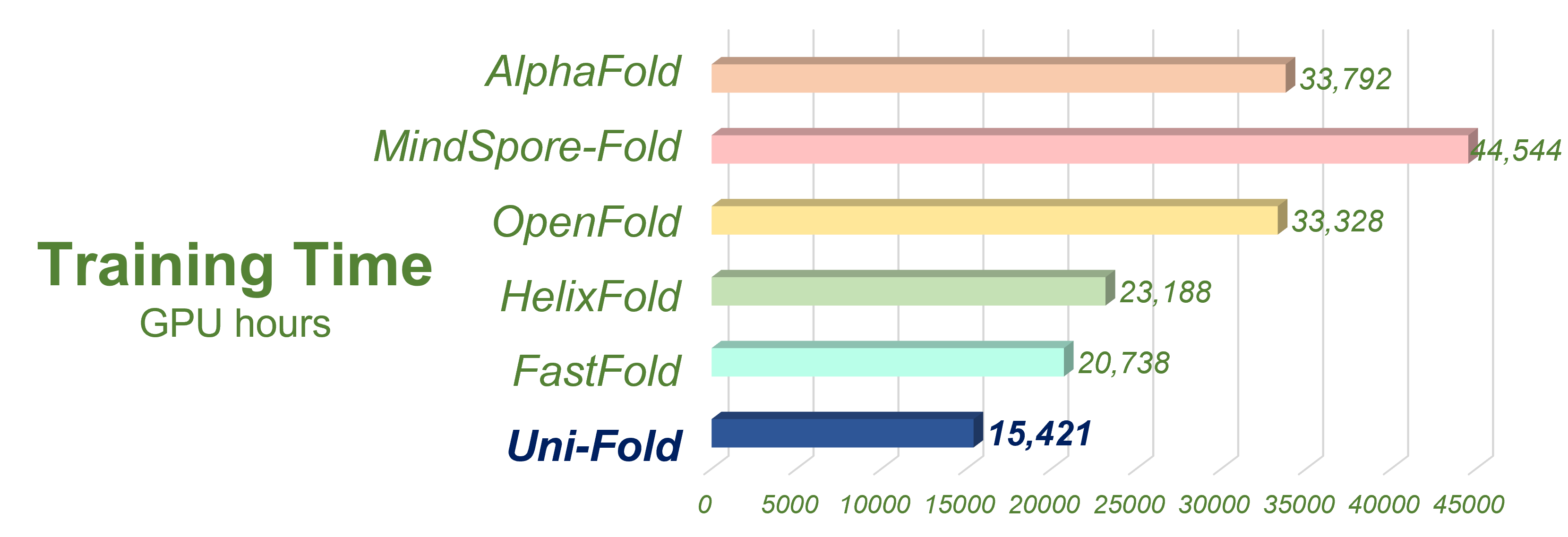
We evaluated Uni-Fold on PDB structures release after our training set with less than 40% template identity. The structures for evaluations are included in evaluation. Uni-Fold enjoys similar monomer prediction accuracy and better multimer prediction accuracy compared with AlphaFold(-Multimer). We also benchmarked the efficiency of Uni-Fold. The end-to-end training efficiency is about 2.2 times of the official AlphaFold. More evaluation results and details are included in our bioRxiv preprint.
Figure 3. Uni-Fold is to our knowledge the fastest implemetation of AlphaFold.
The name Uni-Fold is inherited from our previous repository, Uni-Fold-JAX. First released on Dec 8 2021, Uni-Fold-JAX was the first open-source project (with training scripts) that successfully reproduced the from-scratch training of AlphaFold. Until recently, Uni-Fold-JAX is still the only project that supports training of the original AlphaFold implementation in JAX framework. Due to efficiency and collaboration considerations, we moved from JAX to PyTorch on Jan 2022, based on which we further developed the multimer models.
Uni-Fold is implemented on a distributed PyTorch framework, Uni-Core. As Uni-Core needs to compile CUDA kernels in installation which requires specific CUDA and PyTorch versions, we provide a Docker image to save potential trouble.
To use GPUs within docker you need to install nvidia-docker-2 first. Use the following command to pull the docker image:
docker pull dptechnology/unifold:latest-pytorch1.11.0-cuda11.3Then, you can create and attach into the docker container, and clone & install unifold.
git clone https://github.com/dptech-corp/Uni-Fold
cd Uni-Fold
pip install -e .Training and inference with Uni-Fold require homology searches on sequence and structure databases. Use the following command to download these databases:
bash scripts/download_all_data.sh /path/to/database/directoryMake sure there is at least 3TB storage space for downloading (~500GB) and uncompressing the databases.
Inferenece and finetuning with Uni-Fold requires pretrained model parameters. Use the following command to download the parameters:
wget https://uni-fold.dp.tech/unifold_params_2022-08-01.tar.gz
tar -zxf unifold_params_2022-08-01.tar.gzIt contains 1 monomer and 1 multimer pretrained model parameters, whose model name are model_2_ft and multimer_ft respectively.
One can convert the pretrained AlphaFold and OpenFold parameters to Uni-Fold format via the following commands.
python scripts/convert_alphafold_to_unifold.py \
/path/to/alphafold_params.npz \ # AlphaFold params *.npz file
/path/to/unifold_format.pt \ # save checkpoint in Uni-Fold format
alphafold_model_name \ # specify model name, e.g. model_2_af2, multimer_af2python scripts/convert_openfold_to_unifold.py \
/path/to/openfold_params.pt \ # OpenFold params *.pt file
/path/to/unifold_format.pt \ # save checkpoint in Uni-Fold formatAfter properly configurating the environment and databases, run the following command to predict the structure of the target fasta:
bash run_unifold.sh \
/path/to/the/input.fasta \ # target fasta file
/path/to/the/output/directory/ \ # output directory
/path/to/database/directory/ \ # directory of databases
2020-05-01 \ # use templates before this date
model_name \ # specify model name, must be consistent with model parameters
/path/to/model_parameters.pt # model parametersFor monomer prediction, each fasta file shall contain only one sequence; for multimer prediction, the input fasta file shall contain all sequences of the target complex including duplicated homologous sequences. That is, chains with identical sequences shall be duplicated to their number in the complex.
The output directory of running Uni-Fold contain the predicted structures in *.pdb files, where best.pdb contains prediction with the highest confidence. Besides, other outputs are dumped in *.pkl.gz files. We summarize the confidence metrics, namely plddt and iptm+ptm in *.json files.
Training Uni-Fold relies on pre-calculated features of proteins. We provide a demo dataset in the example data folder. A larger dataset will be released soon.
To start with, we provide a demo script to train the monomer/multimer system of Uni-Fold:
bash train_monomer_demo.sh .and
bash train_multimer_demo.sh .This command starts a training process on the demo data included in this repository. Note that this demo script only tests the correctness of package installation and does not reflect any true performances.
Run the following command to train Uni-Fold Monomer/Multimer from-scratch:
bash train_monomer.sh \ # train_multimer.sh for multimer
/path/to/training/data/directory/ \ # dataset directory
/path/to/output/directory/ \ # output directory where parameters are stored
model_2_af2 # model nameNote that:
- The dataset directory should be configurated in a similar way as the example data.
- The output directory should have enough space to store model parameters (~1.5GB per checkpoint, so empirically 60GB satisfies the default configuration in the shell script).
- We provide several default model names in config.py, namely
model_1,model_2,model_2_af2etc. for monomer models andmultimer,multimer_af2etc. for multimer models. Checkmodel_config()function for the differences between model names. You may also personalize your own model by modifying the function (i.e. forking the if-elses).
Run the following command to finetune the given Uni-Fold Monomer/Multimer parameters:
bash finetune_monomer.sh \ # finetune_multimer.sh for multimer
/path/to/training/data/directory/ \ # dataset directory
/path/to/output/directory/ \ # output directory where parameters are stored
/path/to/pretrained/parameters.pt \ # pretrained parameters
model_2_af2 # model nameBesides the notices in the previous section, additionaly note that:
- The model architecture should be correctly specified by the model name.
- Checkpoints must be in Uni-Fold format (
*.pt).
We provide covenient structure prediction service on Hermite™, a new-generation drug design platform powered by AI, physics, and computing. Users only need to upload sequences of protein monomers and multimers to obtain the predicted structures from Uni-Fold, acompanied by various analyzing tools. Click here for more information of how to use Hermite™.
- Faster Inference with BladeDISC. Alibaba PAI team proposed an end-to-end DynamIc Shape Compiler BladeDISC, which optimized Uni-Fold to achieve faster inference speed and longer sequences inference. For detailed introduction, please see examples in BladeDISC.
If you use the code, the model parameters, the web server at Hermite™, or the released data of Uni-Fold as well as Uni-Fold-JAX, please cite
@article {uni-fold,
author = {Li, Ziyao and Liu, Xuyang and Chen, Weijie and Shen, Fan and Bi, Hangrui and Ke, Guolin and Zhang, Linfeng},
title = {Uni-Fold: An Open-Source Platform for Developing Protein Folding Models beyond AlphaFold},
year = {2022},
doi = {10.1101/2022.08.04.502811},
URL = {https://www.biorxiv.org/content/10.1101/2022.08.04.502811v2},
eprint = {https://www.biorxiv.org/content/10.1101/2022.08.04.502811v2.full.pdf},
journal = {bioRxiv}
}Our training framework is based on Uni-Core. Implementation of fused operators referred to fused_ops and OneFlow. We partly referred to an early version of OpenFold for some of the PyTorch implementations, while mostly followed the original code of AlphaFold. For the data processing part, we followed AlphaFold, and referred to utilities in Biopython, HH-suite3, HMMER, Kalign, pandas, NumPy, and SciPy.
Copyright 2022 DP Technology.
Uni-Fold is licensed under permissive Apache Licence, Version 2.0. You may obtain a copy of the License at https://www.apache.org/licenses/LICENSE-2.0.
The Uni-Fold parameters are made available under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) license. You can find details at: https://creativecommons.org/licenses/by/4.0/legalcode
Uni-Fold is an ongoing project. Our target is to develop better protein folding models and to apply them in real scenarios together with the entire community. We welcome all contributions to this repository, including but not limited to 1) reports and fixes of bugs, 2) new features and 3) accuracy and efficiency improvements. Please refer to CONTRIBUTING.md for more information.
Use of the third-party software, libraries or code referred to in the Acknowledgements section above may be governed by separate terms and conditions or license provisions. Your use of the third-party software, libraries or code is subject to any such terms and you should check that you can comply with any applicable restrictions or terms and conditions before use.