A tool to detect and visualize target gene fusions by scanning FASTQ files directly. This tool accepts FASTQ files and reference genome as input, and outputs detected fusion results in TEXT, JSON and HTML formats.
- Sample HTML report: http://opengene.org/GeneFuse/report.html
- Sample JSON report: http://opengene.org/GeneFuse/report.json
- Dataset for testing: http://opengene.org/dataset.html Please download the paired-end FASTQ files for GeneFuse testing (Illumina platform)
conda install -c bioconda genefuseThis binary is only for Linux systems, http://opengene.org/GeneFuse/genefuse
# this binary was compiled on CentOS, and tested on CentOS/Ubuntu
wget http://opengene.org/GeneFuse/genefuse
chmod a+x ./genefuse# get source (you can also use browser to download from master or releases)
git clone https://github.com/OpenGene/genefuse.git
# build
cd genefuse
make
# Install
sudo make installYou should provide following arguments to run genefuse
- the reference genome fasta file, specified by
-ror--ref= - the fusion setting file, specified by
-for--fusion= - the fastq file(s), specified by
-1or--read1=for single-end data. If dealing with pair-end data, specify the read2 file by-2or--read2= - use
-hor--html=to specify the file name of HTML report - use
-jor--json=to specify the file name of JSON report - the plain text result is directly printed to STDOUT, you can pipe it to a file using a
>
genefuse -r hg19.fasta -f genes/druggable.hg19.csv -1 genefuse.R1.fq.gz -2 genefuse.R2.fq.gz -h report.html > resultThe reference genome should be a single whole FASTA file containg all chromosome data. This file shouldn't be compressed. For human data, typicall hg19/GRch37 or hg38/GRch38 assembly is used, which can be downloaded from following sites:
hg19/GRch37: ftp://ftp.ncbi.nlm.nih.gov/sra/reports/Assembly/GRCh37-HG19_Broad_variant/Homo_sapiens_assembly19.fastahg38/GRch38: http://hgdownload.cse.ucsc.edu/goldenPath/hg38/bigZips/hg38.fa.gz Remember to decompress hg38.fa.gz since it is gzipped and is not supported currently.
The fusion file is a list of coordinated target genes together with their exons. A sample is:
>EML4_ENST00000318522.5,chr2:42396490-42559688
1,42396490,42396776
2,42472645,42472827
3,42483641,42483770
4,42488261,42488434
5,42490318,42490446
...
>ALK_ENST00000389048.3,chr2:29415640-30144432
1,30142859,30144432
2,29940444,29940563
3,29917716,29917880
4,29754781,29754982
5,29606598,29606725
...
The coordination system should be consistent with the reference genome.
Four fusion files are provided with genefuse:
genes/druggable.hg19.csv: all druggable fusion genes based onhg19/GRch37reference assembly.genes/druggable.hg38.csv: all druggable fusion genes based onhg38/GRch38reference assembly.genes/cancer.hg19.csv: all COSMIC curated fusion genes (http://cancer.sanger.ac.uk/cosmic/fusion) based onhg19/GRch37reference assembly.genes/cancer.hg38.csv: all COSMIC curated fusion genes (http://cancer.sanger.ac.uk/cosmic/fusion) based onhg38/GRch38reference assembly.
Notes:
genefuseruns almost ~5X faster withdruggablegenes thancancergenes, sincedruggablegenes are only a small subset ofcancergenes. Use this one if you only care about the fusion related personalized medicine for cancers.- The
cancergenes should be enough for most cancer related studies, since all COSMIC curated fusion genes are included. - If you want to create a custom gene list, please follow the instructions given on next section.
If you'd like to create a custom fusion file, you can use scripts/gen_fusion_file.jl, which is based on the Julia library OpenGene.jl to generate the fusion file you want.
You should prepare a file containing all genes you want, seperated by space or line break. Please note that comma is not supported. Each gene should be the HGNC standard name.
By default, the primary transcript (named as GENE_001) will be used. But you can specify the transcript by add _TranscriptId to the gene. For example: use CD74_ENST00000009530 to specify the transcript of CD74.
When the gene list file (genes.txt) is prepared, you can used following command to generate a fusion file (fusion.csv):
julia scripts/gen_fusion_file.jl -r hg19 -g genes.txt -f fusion.csvThe reference genome is specified by -r option, which can be hg19/GRch37/GRch38.
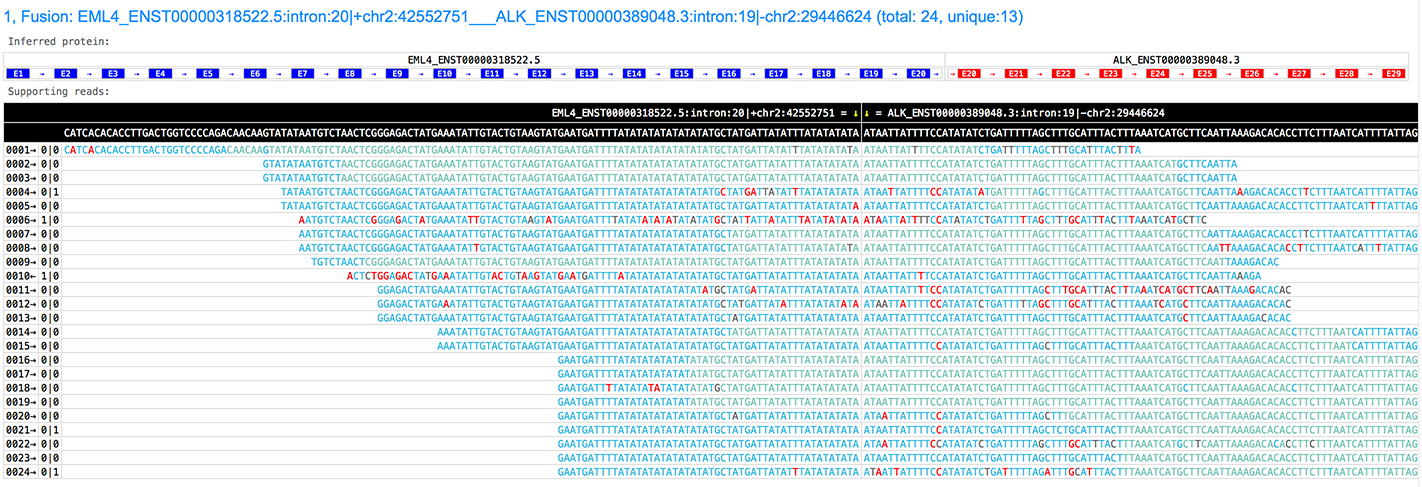
GeneFuse can generate very informative and interactive HTML pages to visualize the fusions with following information:
- the fusion genes, along with their transcripts.
- the inferred break point with reference genome coordinations.
- the inferred fusion protein, with all exons and the transcription direction.
- the supporting reads, with all bases colorized according to their quality scores.
- the number of supporting reads, and how many of them are unique (the rest may be duplications)
See the HTML page of this picture: http://opengene.org/GeneFuse/report.html
options:
-1, --read1 read1 file name (string)
-2, --read2 read2 file name (string [=])
-f, --fusion fusion file name, in CSV format (string)
-r, --ref reference fasta file name (string)
-u, --unique specify the least supporting read number is required to report a fusion, default is 2 (int [=2])
-d, --deletion specify the least deletion length of a intra-gene deletion to report, default is 50 (int [=50])
-h, --html file name to store HTML report, default is genefuse.html (string [=genefuse.html])
-j, --json file name to store JSON report, default is genefuse.json (string [=genefuse.json])
-t, --thread worker thread number, default is 4 (int [=4])
-?, --help print this message
If you used GeneFuse in you work, you can cite it as:
Shifu Chen, Ming Liu, Tanxiao Huang, Wenting Liao, Mingyan Xu and Jia Gu. GeneFuse: detection and visualization of target gene fusions from DNA sequencing data. International Journal of Biological Sciences, 2018; 14(8): 843-848. doi: 10.7150/ijbs.24626