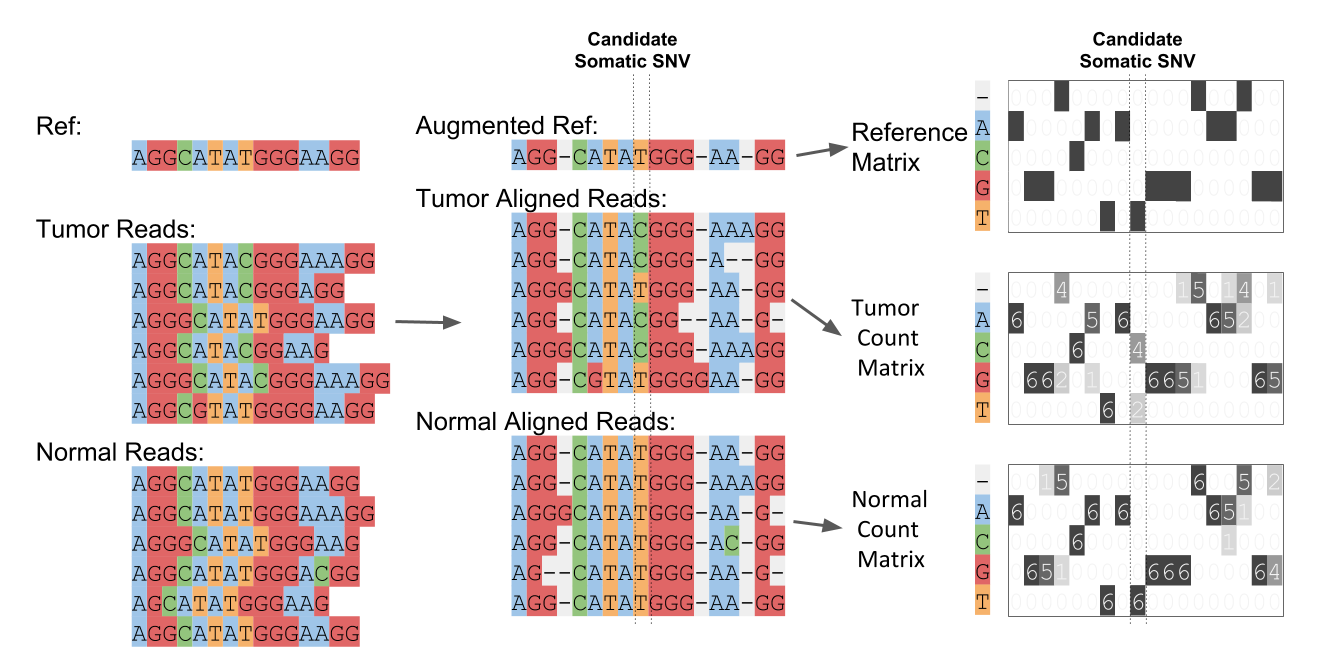
NeuSomatic is based on deep convolutional neural networks for accurate somatic mutation detection. With properly trained models, it can robustly perform across sequencing platforms, strategies, and conditions. NeuSomatic summarizes and augments sequence alignments in a novel way and incorporates multi-dimensional features to capture variant signals effectively. It is not only a universal but also accurate somatic mutation detection method.
For more information contact us at bioinformatics.red@roche.com
If you use NeuSomatic in your work, please cite the following papers:
Sayed Mohammad Ebrahim Sahraeian, Ruolin Liu, Bayo Lau, Karl Podesta, Marghoob Mohiyuddin, Hugo Y. K. Lam,
Deep convolutional neural networks for accurate somatic mutation detection. Nature Communications 10: 1041, (2019).
doi: https://doi.org/10.1038/s41467-019-09027-x
Sayed Mohammad Ebrahim Sahraeian, Li Tai Fang, Marghoob Mohiyuddin, Huixiao Hong, Wenming Xiao,
Robust cancer mutation detection with deep learning models derived from tumor-normal sequencing data. bioRxiv (2019): 667261.
doi: https://doi.org/10.1101/667261
Availability
NeuSomatic Docker Image
Required Inputs
Quick Test
Example Usage
Ensemble mode
Creating Training Data
Trained Network Models
HPC run
License
NeuSomatic is written in Python and C++ and requires a Unix-like environment to run. It has been sucessfully tested on CentOS 7. Its deep learning framework is implemented using PyTorch 1.1.0 to enable GPU acceleration for training/testing.
NeuSomatic first scans the genome to identify candidate variants and extract alignment information.
The binary for this step can be obtained at neusomatic/bin folder by running ./build.sh (which requires cmake 3.13.2 and g++ 5.4.0).
Python 3.7 and the following Python packages must be installed:
- pytorch 1.1.0
- torchvision 0.3.0
- pybedtools 0.8.0
- pysam 0.15.2
- zlib 1.2.11
- numpy 1.15.4
- scipy 1.2.0
- imageio 2.5.0
- biopython 1.73
It also depends on the following packages:
- cudatoolkit 9.0 (if you want to use GPU)
- tabix 0.2.6
- bedtools 2.27.1
- samtools 1.9
You can install these packages using anaconda/miniconda :
conda install zlib=1.2.11 numpy=1.15.4 scipy=1.2.0 cmake=3.13.2 imageio=2.5.0
conda install pysam=0.15.2 pybedtools=0.8.0 samtools=1.9 tabix=0.2.6 bedtools=2.27.1 biopython=1.73 -c bioconda
conda install pytorch=1.1.0 torchvision=0.3.0 cudatoolkit=9.0 -c pytorch
Then you can export the conda paths as:
export PATH="/PATH/TO/CONDA/bin:$PATH"
export LD_LIBRARY_PATH="/PATH/TO/CONDA/lib:$LD_LIBRARY_PATH"
g++ 5.4.0 can also be obained as sudo apt-get install gcc-5 g++-5.
The docker image with all the packages installed (CPU-only) can be found at https://hub.docker.com/r/msahraeian/neusomatic/
To use GPU (in train.py and call.py steps), you should use conda environment to locally install required packages as shown above.
The dockerfile is also available at docker/Dockerfile for local build.
Examples on how to use the docker image are shown at test/docker_test.sh.
For training mode, the following inputs are required:
- tumor
.bamalignment file - normal
.bamalignment file - training region
.bedfile - truth somatic variant
.vcffile
For calling mode, the following inputs are required:
- tumor
.bamalignment file - normal
.bamalignment file - call region
.bedfile - trained model
.pthfile
For the region .bed files, if you don't have any preferred target regions for training/calling, you can use the whole genome as the target region. Example bed files of major chromosomes for human hg38, hg19, and b37 references can be found at resources.
Testing the preprocessing, calling, and postprocessing steps:
cd test
./run_test.sh
The outputs at test/example/work_standalone/NeuSomatic_standalone.vcf and test/example/work_ensemble/NeuSomatic_ensemble.vcf for stand-alone and ensemble modes should look like test/NeuSomatic_standalone.vcf and test/NeuSomatic_ensemble.vcf, respectively.
Similarly, you can test docker image as:
cd test
./docker_test.sh
For training:
- Preprocess step in train mode (scan the alignments, find candidates, generate input matrix dataset)
python preprocess.py \
--mode train \
--reference GRCh38.fa \
--region_bed region.bed \
--tumor_bam tumor.bam \
--normal_bam normal.bam \
--work work_train \
--truth_vcf truth.vcf \
--min_mapq 10 \
--number_threads 10 \
--scan_alignments_binary ../bin/scan_alignments
- Train network
python train.py \
--candidates_tsv work_train/dataset/*/candidates*.tsv \
--out work_train \
--num_threads 10 \
--batch_size 100
If you want to continue training starting from a pretrained model you can use --checkpoint.
For testing:
- Preprocess step in call mode (scan the alignments, find candidates, generate input matrix dataset)
python preprocess.py \
--mode call \
--reference GRCh38.fa \
--region_bed region.bed \
--tumor_bam tumor.bam \
--normal_bam normal.bam \
--work work_call \
--min_mapq 10 \
--number_threads 10 \
--scan_alignments_binary ../bin/scan_alignments
- Call variants
python call.py \
--candidates_tsv work_call/dataset/*/candidates*.tsv \
--reference GRCh38.fa \
--out work_call \
--checkpoint work_train/some_checkpoint.pth \
--num_threads 10 \
--batch_size 100
- Postprocess step (resolve long INDEL sequences, report vcf)
python postprocess.py \
--reference GRCh38.fa \
--tumor_bam tumor.bam \
--pred_vcf work_call/pred.vcf \
--candidates_vcf work_call/work_tumor/filtered_candidates.vcf \
--output_vcf work_call/NeuSomatic.vcf \
--work work_call
Here, the final NeuSomatic prediction is reported at work_call/NeuSomatic.vcf.
NeuSomatic will use GPUs in train/call steps if they are avilable. To use specific GPUs for train/call steps, you can set the environment variable CUDA_VISIBLE_DEVICES as:
CUDA_VISIBLE_DEVICES=0,1,2,3 python train.py ...
or
CUDA_VISIBLE_DEVICES=0,1,2,3 python call.py ...
To run in CPU mode you can disable accessing to GPU by exporting CUDA_VISIBLE_DEVICES=.
NeuSomatic can be used universally as a stand-alone somatic mutation detection method or with an ensemble of existing methods. NeuSomatic currently supports outputs from MuTect2, MuSE, Strelka2, SomaticSniper, VarDict, and VarScan2. For ensemble mode, the ensembled outputs of different somatic callers (as a single .tsv file) should be prepared and inputed using --ensemble_tsv argument in preprocess.py and postprocess.py .
There are two alternative ways to prepare this file:
-
Dockerized solution for running all of the individual somatic callers (MuTect2, MuSE, Strelka2, SomaticSniper, VarDict, and VarScan2), and a wrapper that combines their output is explained at ensemble_docker_pipelines.
-
Alternartively, if you don't want to use docker and already have the output of all somatic callers, you can prepare the
.tsvfile using theSomaticSeq.Wrapper.shscript here (you may need to install the necessary dependencies for this script, explained here).For instance:
SomaticSeq.Wrapper.sh \ --output-dir output \ --genome-reference GRCh38.fa \ --tumor-bam tumor.bam \ --normal-bam normal.bam \ -mutect2 MuTect2.vcf \ --varscan-snv VarScan2.snp.vcf \ --varscan-indel VarScan2.indel.vcf \ -sniper SomaticSniper.vcf \ --vardict VarDict.vcf \ --muse MuSE.vcf \ --strelka-snv somatic.snvs.vcf.gz \ --strelka-indel somatic.indels.vcf.gz \ --inclusion-region region.bed \ -dbsnp dbsnp.GRCh38.vcf \ -gatk GenomeAnalysisTK.jarThen, in the output directory, do:
cat <(cat Ensemble.s*.tsv |grep CHROM|head -1) \ <(cat Ensemble.s*.tsv |grep -v CHROM) | sed "s/nan/0/g" > ensemble_ann.tsvand provide
ensemble_ann.tsvas--ensemble_tsvargument inpreprocess.pyandpostprocess.py.
- To train or call in the ensemble mode you should use
--ensembleargument intrain.pyandcall.py.
The best performance can be obtained when the network is trained on your input dataset. You can creat training data for your input data using BAMSurgeon that can spike in in silico somatic mutations into existing BAM files. The dockerized piplines and complete documentation on how to creat training sets can be found here.
You can then used the synthetic tumor/normal pair and the known in silico spiked mutations (as truth set) to train the NeuSomatic network as shown in Example Usage.
We provide a set of trained NeuSomatic network models for general purpose usage. Users should note that these models are trained for sepcific settings and are not supposed to work perfectly for all circumestances.
The SEQC-II pretrained models are the recommended NeuSomatic models and are analyzed in detail in Sahraeian et al. 2019.
The following models can be found at neusomatic/models folder:
| Model | Mode | Training Information |
|---|---|---|
NeuSomatic_v0.1.4_standalone_SEQC-WGS-Spike.pth |
Stand-alone | SEQC-II (SEQC-WGS-Spike model) (trained on 20 WGS replicate pairs with in silico somatic mutations of 1%-100% AF, matched with both 95%N and 100%N, Illumina HiSeq and NovaSeq, BWA-MEM, ~40x-220x) |
NeuSomatic_v0.1.4_ensemble_SEQC-WGS-Spike.pth |
Ensemble | SEQC-II (SEQC-WGS-Spike model) (trained on 20 WGS replicate pairs with in silico somatic mutations of 1%-100% AF, matched with both 95%N and 100%N, Illumina HiSeq and NovaSeq, BWA-MEM, ~40x-220x, 5 callers used: MuTect2, Strelka2, MuSE, SomaticSniper, VarDict) |
NeuSomatic_v0.1.4_standalone_SEQC-WGS-GT50-SpikeWGS10.pth |
Stand-alone | SEQC-II (SEQC-WGS-GT50-SpikeWGS10 model) (trained on combination of two datasets: (1) 50% of the genome for 24 real tumor-normal SEQC-II replicates using the HighConf truth set annotation, with multiple purity settings of 100T-100N/10T-100N/10T-95N, 1%-100% AF and (2) 10% of data used in NeuSomatic_v0.1.4_standalone_SEQC-WGS-Spike.pth model. Illumina HiSeq and NovaSeq, BWA-MEM, ~40x-390x) |
NeuSomatic_v0.1.4_ensemble_SEQC-WGS-GT50-SpikeWGS10.pth |
Ensemble | SEQC-II (SEQC-WGS-GT50-SpikeWGS10 model) (trained on combination of two datasets: (1) 50% of the genome for 24 real tumor-normal SEQC-II replicates using the HighConf truth set annotation, with multiple purity settings of 100T-100N/10T-100N/10T-95N, 1%-100% AF and (2) 10% of data used in NeuSomatic_v0.1.4_ensemble_SEQC-WGS-Spike.pth model. Illumina HiSeq and NovaSeq, BWA-MEM, ~40x-390x, 5 callers used: MuTect2, Strelka2, MuSE, SomaticSniper, VarDict) |
| Model | Mode | Training Information |
|---|---|---|
NeuSomatic_v0.1.3_standalone_Dream3.pth |
Stand-alone | WGS Dream Challenge Stage 3 (trained on multiple purity settings: 100T-100N/50T-100N/70T-95N/50T-95N/25T-95N, Illumina, BWA-MEM, ~30x) |
NeuSomatic_v0.1.3_ensemble_Dream3.pth |
Ensemble | WGS Dream Challenge Stage 3 (trained on multiple purity settings: 100T-100N/50T-100N/70T-95N/50T-95N/25T-95N, Illumina, BWA-MEM, ~30x, 6 callers used: MuTect2, Strelka2, MuSE, SomaticSniper, VarDict, VarScan2) |
NeuSomatic_v0.1.0_standalone_Dream3_70purity.pth |
Stand-alone | WGS Dream Challenge Stage 3 (70% tumor and 95% normal purities, Illumina, BWA-MEM, ~30x) |
NeuSomatic_v0.1.0_ensemble_Dream3_70purity.pth |
Ensemble | WGS Dream Challenge Stage 3 (70% tumor and 95% normal purities, Illumina, BWA-MEM, ~30x, 6 callers used: MuTect2, Strelka2, MuSE, SomaticSniper, VarDict, VarScan2) |
NeuSomatic_v0.1.0_standalone_WEX_100purity.pth |
Stand-alone | WEX (100% tumor and normal purities, Illumina, BWA-MEM, ~125x) |
NeuSomatic_v0.1.0_ensemble_WEX_100purity.pth |
Ensemble | WEX (100% tumor and normal purities, Illumina, BWA-MEM, ~125x, 6 callers used: MuTect2, Strelka2, MuSE, SomaticSniper, VarDict, VarScan2) |
For distributed data processing on HPC platforms, the input regions can be splitted to smaller sub-regions as follows:
python split_bed.py \
--region_bed region.bed \
--output work \
--num_splits 10
Then, each sub-region can be processed as follows (for instance on SGE cluster):
for i in {0..10}
do
qsub -pe smp 24 \
"python preprocess.py \
--mode call [or --mode train]
--reference GRCh38.fa --tumor_bam tumor.bam --normal_bam normal.bam \
--region_bed work/splits/region_${i}.bed \
--work work/work_${i} \
--min_mapq 10 --number_threads 24 \
--scan_alignments_binary ../bin/scan_alignments"
done
Then the candiate .tsv files to be used for train/call can be found at work_*/dataset/*/candidates*.tsv.
NeuSomatic is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.