This file contains background information and instructions for the final project.
Alzheimer's disease (AD) is a progressive neurodegenerative disorder that results in impaired neuronal (brain cell) function and eventually, cell death. AD is the most common cause of dementia. Clinically, it is characterized by memory loss, inability to learn new material, loss of language function, and other manifestations.
For patients exhibiting early symptoms, quantifying disease progression over time can help direct therapy and disease management.
A radiological study via MRI exam is currently one of the most advanced methods to quantify the disease. In particular, the measurement of hippocampal volume has proven useful to diagnose and track progression in several brain disorders, most notably in AD. Studies have shown reduced volume of the hippocampus in patients with AD.
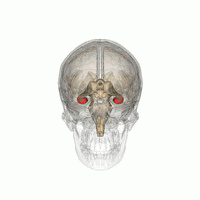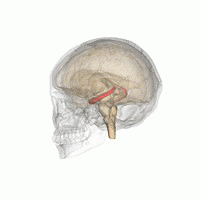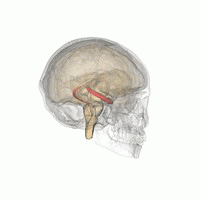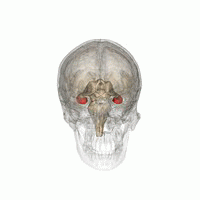
The hippocampus is a critical structure of the human brain (and the brain of other vertebrates) that plays important roles in the consolidation of information from short-term memory to long-term memory. In other words, the hippocampus is thought to be responsible for memory and learning (that's why we are all here, after all!)
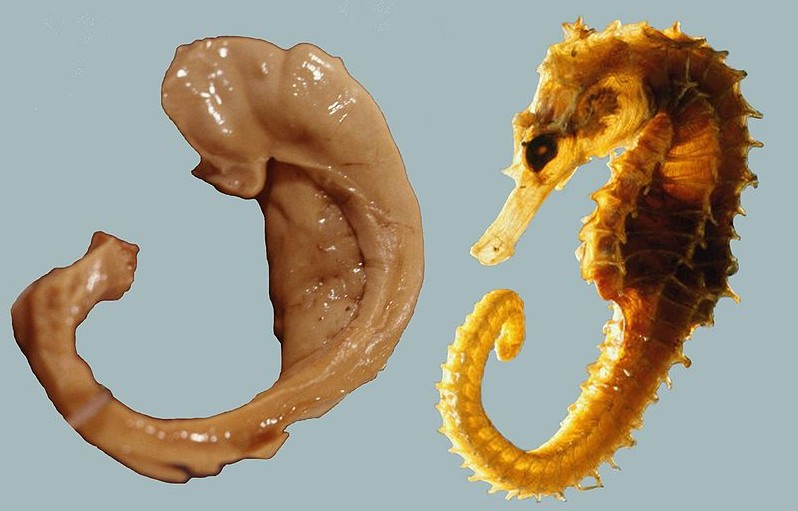
Humans have two hippocampi, one in each hemishpere of the brain. They are located in the medial temporal lobe of the brain. Fun fact - the word "hippocampus" is roughly translated from Greek as "horselike" because of the similarity to a seahorse, a peculiarity observed by one of the first anatomists to illustrate the structure.
According to studies, the volume of the hippocampus varies in a population, depending on various parameters, within certain boundaries, and it is possible to identify a "normal" range when taking into account age, sex and brain hemisphere.
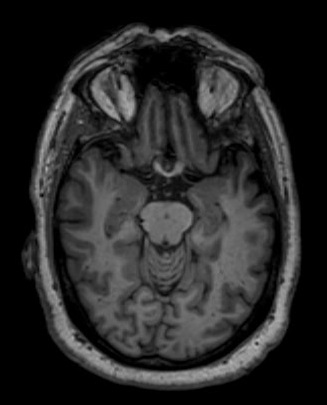
There is one problem with measuring the volume of the hippocampus using MRI scans, though - namely, the process tends to be quite tedious since every slice of the 3D volume needs to be analyzed, and the shape of the structure needs to be traced. The fact that the hippocampus has a non-uniform shape only makes it more challenging. Do you think you could spot the hippocampi in this axial slice?
As you might have guessed by now, we are going to build a piece of AI software that could help clinicians perform this task faster and more consistently.
You have seen throughout the course that a large part of AI development effort is taken up by curating the dataset and proving clinical efficacy. In this project, we will focus on the technical aspects of building a segmentation model and integrating it into the clinician's workflow, leaving the dataset curation and model validation questions largely outside the scope of this project.
In this project you will build an end-to-end AI system which features a machine learning algorithm that integrates into a clinical-grade viewer and automatically measures hippocampal volumes of new patients, as their studies are committed to the clinical imaging archive.
Fortunately you won't have to deal with full heads of patients. Our (fictional) radiology department runs a HippoCrop tool which cuts out a rectangular portion of a brain scan from every image series, making your job a bit easier, and our committed radiologists have collected and annotated a dataset of relevant volumes, and even converted them to NIFTI format!
You will use the dataset that contains the segmentations of the right hippocampus and you will use the U-Net architecture to build the segmentation model.
After that, you will proceed to integrate the model into a working clinical PACS such that it runs on every incoming study and produces a report with volume measurements.
We are using the "Hippocampus" dataset from the Medical Decathlon competition. This dataset is stored as a collection of NIFTI files, with one file per volume, and one file per corresponding segmentation mask. The original images here are T2 MRI scans of the full brain. As noted, in this dataset we are using cropped volumes where only the region around the hippocampus has been cut out. This makes the size of our dataset quite a bit smaller, our machine learning problem a bit simpler and allows us to have reasonable training times. You should not think of it as "toy" problem, though. Algorithms that crop rectangular regions of interest are quite common in medical imaging. Segmentation is still hard.
You will have two options for the environment to use throughout this project:
[Christa to put some canned common text about Udacity Workspaces]
If you would like to run the project locally, you would need a Python 3.7+ environment with the following libraries for the first two sections of the project:
- nibabel
- matplotlib
- numpy
- pydicom
- PIL
- json
- torch (preferably with CUDA)
- tensorboard
In the 3rd section of the project we will be working with three software products for emulating the clinical network. You would need to install and configure:
- Orthanc server for PACS emulation
- OHIF zero-footprint web viewer for viewing images. Note that if you deploy OHIF from its github repository, at the moment of writing the repo includes a yarn script (
orthanc:up) where it downloads and runs the Orthanc server from a Docker container. If that works for you, you won't need to install Orthanc separately. - If you are using Orthanc (or other DICOMWeb server), you will need to configure OHIF to read data from your server. OHIF has instructions for this: https://docs.ohif.org/configuring/data-source.html
- In order to fully emulate the Udacity workspace, you will also need to configure Orthanc for auto-routing of studies to automatically direct them to your AI algorithm. For this you will need to take the script that you can find at
section3/src/deploy_scripts/route_dicoms.luaand install it to Orthanc as explained on this page: https://book.orthanc-server.com/users/lua.html - DCMTK tools for testing and emulating a modality. Note that if you are running a Linux distribution, you might be able to install dcmtk directly from the package manager (e.g.
apt-get install dcmtkin Ubuntu)
You will perform this section in the Workspace 1. This workspace has a Python virtual environment called medai which is set up with everything that you need to train your ML model. This workspace also has a GPU which will speed up your training process quite significantly.
The data is located in /data/TrainingSet directory here.
In the project directory called section1 you will find a Python Notebook that has a few instructions in it that will help you inspect the dataset, understand the clinical side of the problem a bit better, and get it ready for consumption by your algorithm in Section 2. The notebook has 2 types of comments:
- Comments marked with
# TASK:are tasks, instructions, or questions you have to complete. - Comments not marked are not mandatory but are suggestions, questions, or background that will help you get a better understanding of the subject and apply your newly acquired medical imaging dataset EDA skills.
Navigate to the directory section1/out to find the README.md with instructions on what is expected as the outcome.
You will perform this section in the same workspace as Section 1: Workspace 1. This workspace has a Python virtual environment called medai [TODO: how are we doing this?] which is set up with everything that you need to analyze inspect the dataset and prepare it for machine learning.
In the directory called section2/src you will find the source code that forms the framework for your machine learning pipeline.
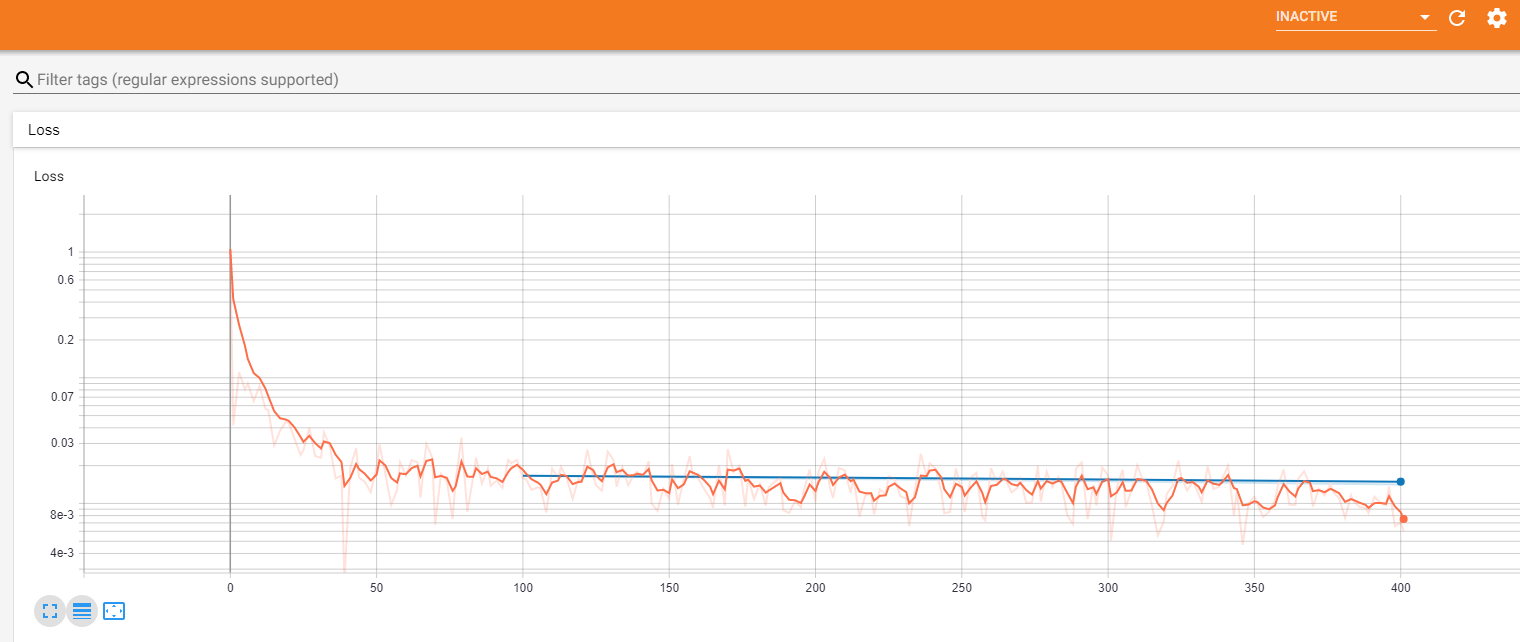
You will be using PyTorch to train the model, similar to our Segmentation&Classification Lesson, and we will be using Tensorboard to visualize the results.
You will use the script run_ml_pipeline.py to kick off your training pipeline. You can do so right now! The script will not get far, though. It only contains the skeleton of the final solution and a lot of comments. You will need to follow the instructions inside the code files to complete the section and train your model. Same convention is used as in Section 1:
- Comments that start with
# TASKare tasks, instructions, or questions you have to complete - All other types of comments provide additional background, questions or contain suggestions to make your project stand out.
You will need to complete all the instructional comments in the code in order to complete this section. You can do this in any order, but it makes most sense to start with the code in run_ml_pipeline.py.
The code has hooks to log progress to Tensorboard. In order to see the Tensorboard output you need to launch Tensorboard executable from the same directory where run_ml_pipeline.py is located using the following command:
tensorboard --logdir runs --bind_all
After that, Tensorboard will write logs into directory called runs and you will be able to view progress by opening the browser and navigating to default port 6006 of the machine where you are running it.
Navigate to the directory section2/out to find the README.md with instructions on what is expected as the outcome.
In this final section you will use some of the work you did for Section 2 to create an AI product that can be integrated into a clinical network and provide the auto-computed information on the hippocampal volume to the clinicians. While hospital integrations are typically handled by hospital IT staff, it will help tremendously if you can talk the same language with the people who will operate your model, and will have a feel for how clinical radiological software works. These skills will also help you debug your model in the field.
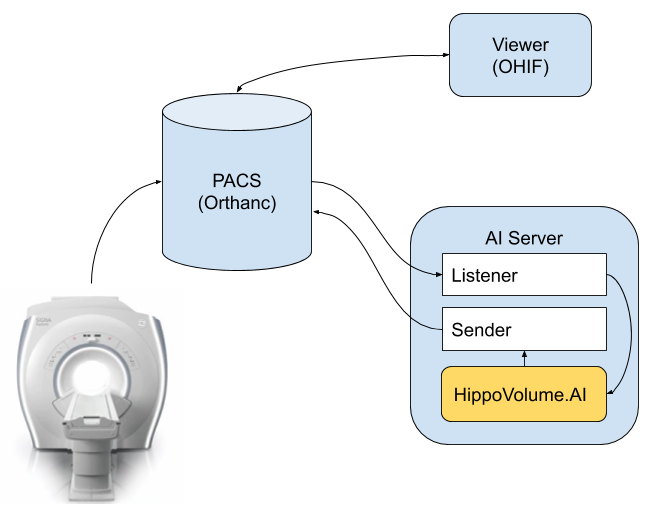
You will perform this section in a different workspace than the previous two sections: Workspace 2. This workspace is a simpler hardware, with no GPU, which is more representative of a clinical environment. This workspace also has a few tools installed in it, which is replicates the following clinical network setup:
Specifically, we have the following software in this setup:
- MRI scanner is represented by a script
section3/src/deploy_scripts/send_volume.sh. When you run this script it will simulate what happens after a radiological exam is complete, and send a volume to the clinical PACS. Note that scanners typically send entire studies to archives. - PACS server is represented by Orthanc deployment that is listening to DICOM DIMSE requests on port 4242. Orthanc also has a DicomWeb interface that is exposed at port 8042, prefix /dicom-web. There is no authentication and you are welcome to explore either one of the mechanisms of access using a tool like curl or Postman. Our PACS server is also running an auto-routing module that sends a copy of everything it receives to an AI server. See instructions ad the end of this page on how to launch if you are using the Udacity Workspace.
- Viewer system is represented by OHIF. It is connecting to the Orthanc server using DicomWeb and is serving a web application on port 3000. Again, see instructions at the end of this page if you are using the Udacity Workspace.
- AI server is represented by a couple of scripts.
section3/src/deploy_scripts/start_listener.shbrings up a DCMTK'sstorescpand configures it to just copy everything it receives into a directory that you will need to specify by editing this script, organizing studies as one folder per study. HippoVolume.AI is the AI module that you will create in this section.
If you want to replicate this environment on your local machine, you will find instructions in the Project Overview concept.
As with Section 2, in the directory called section3/src you will find the source code that forms the skeleton of the HippoVolume.AI module.
inference_dcm.py is the file that you will be working on. It contains code that will analyze the directory of the AI server that contains the routed studies, find the right series to run your algorithm on, will generate report, and push it back to our PACS.
Note that in real system you would architect things a bit differently. Probably, AI server would be a separate piece of software that would monitor the output of the listener, and would manage multiple AI modules, deciding which one to run, automatically. In our case, for the sake of simplicity, all code sits in one Python script that you would have to run manually after you simulate an exam via the send_volume.sh script - inference_dcm.py. It combines the functions of processing of the listener output and executing the model, and it does not do any proper error handling :)
As before, you will need to follow the instructions inside the code files to complete the section and create your AI module. Same convention is used as in Sections 1 and 2: comments that start with # TASK instruct you to create certain code snippets, and all other types of comments provide background or stand-out suggestions.
You will need to complete all the instructional comments in the code in order to complete this section. You can do this in any order, but it makes most sense to start with the code in inference_dcm.py.
Once you complete the code, you can test it by running
deploy_scripts/send_volume.sh
which will simulate a completion of MRI study and sending of patient data to our PACS, and then following that by running inference_dcm.py
The send_volume.sh script needs to be run from directory section3/src (because it relies on relative paths). If you did everything correctly, an MRI scan will be sent to the PACS and to your module which will compute the volume, prepare the report and push it back to the PACS so that it could be inspected in our clinical viewer.
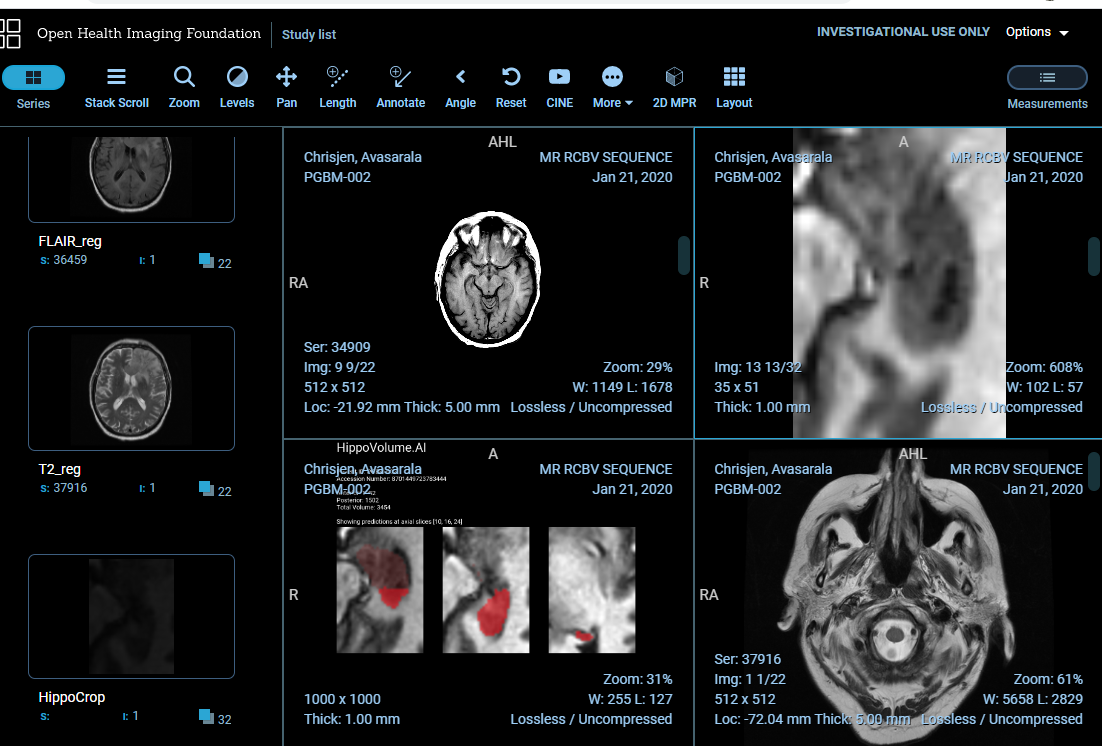
At this point, go to [YOUR IP ADDRESS]:3000 (can be another port if you are using Udacity Workspace) which brings up our OHIF viewer. You should be able to inspect your report in all its glory, in the context of a radiological study presented to a radiologist in a clinical viewer!
The study that send_result.sh sends, and a few other sample studies are located in /data/TestVolumes. Feel free to modify the script to try out your algorithm with other volumes.
Note, that the DICOM studies used for inferencing this section have been created artificially, and while full-brain series belong to the same original study, this is not the study from which the hippocampus crop is taken.
Now that you have built a radiological AI system and given it to clinicians, you can start collecting data on how your model performs in the real world. If you (or the company you work for) intends to commercialize your technology, you will have to clear the regulatory bar. As we have discussed in our final lesson, an important contribution of an AI engineer to this endeavor is helping execute the clinical validation by contributing to a validation plan. Your final task in this course is to write a draft of such plan (shoot for 1-2 pages for this exercise). Remember - clinical validation is all about proving that your technology performs the way you claim it does. If you are saying that it can measure hippocampal volume, your validation needs prove that it actually does, and establish the extents to which your claim is true. Your validation plan needs to define how you would prove this, and establish these extents.
For the purpose of this exercise, assume that you have access to any clinical facility and patient cohorts you need, and that you have all the budget in the world. Assume that you know where your data came from and that you know how to label it (just come up with a good story). In your plan, touch on at least the following:
- Your algorithm relies upon certain "ground truth" - how did you define your ground truth? How will you prove that your method of collecting the ground truth is robust and represents the population that you claim this algorithm is good for?
- How do you define accuracy of your algorithm and how do you measure it with respect to real world population? Check out the calculator and report from HippoFit for some inspiration.
- How do you define what data your algorithm can operate on?
There is no right answer here - think of these and other questions that would come up during validation of such algorithm. Thinking of such things early on will help you build better algorithms in the first place.
Navigate to the directory section3/out to find the README.md with instructions on what is expected as the outcome.
If you were able to get here after completing all the tasks above - congratulations! You have gone through the challenging process of integrating knowledge of clinical context, data analysis, machine learning systems, and medical imaging networking to create a fully functional AI module for a radiological system.
Armed with this knowledge you will be able to get quickly started with a vast majority of problems in 3D radiological imaging space, and even transfer this knowledge over to non-radiological modalities that generate 3D images.
At the moment of writing in 2020, medical imaging AI is a very rapidly growing space, and the potential of the field is staggering. We are only starting to get access to good clinical datasets, the ImageNets of medical imaging is yet to come, clinician researchers are just starting to wrap their heads around what is possible with machine-learning-based technology and tools are becoming better every day. Information flow between data scientists and clinicians is key to unlocking the potential of medical AI and helping clinicians reduce the amount of mundane work, become more precise, efficient, and less stressed. This is just the beginning.
If you are curious to learn more about the space and see what others are doing, here are a few useful resources, companies and societies to watch for.
- MICCAI Society hosts an annual conference dedicated to medical imaging and related fields, and also hosts a number of challenges. One that has consistently generated good volumetric datasets is called BRATS
- Radiological Society of North America is a renowned organization that unifies medical imaging professionals around the globe. In addition to hosting the eponymous largest medical imaging conference in the world it has been turning more attention to AI recently, and hosted interesting medical imaging competitions within its "AI challenge" program. Last year's challenged featured a classification problem for CT imaging (although with the focus on 2D methods)
- SIIM is a society that focuses on medical imaging informatics and it has recently started running a machine learning sub-conference called C-MIMI
It wouldn't be much of an overstatement to say that almost every academic medical center in the world is running some sort of a medical imaging AI program. These are all very interesting since they are rooted in clinical expertise and benefit from access to data. They vary in size and often are a part of larger, disease-specific programs. A couple efforts worthy of noting are:
- Center for Clinical Data Science by Parthers Healthcare
- Stanford's AIMI
- National Consortium of Intelligent Medical Imaging, kicked off by the University of Oxford and the UK's National Health Service
There are plenty and there will be more. Some choose to pursue a clinical workflow, some focus on application of particular machine learning technique and some capitalize on existing clinical footprint and invest in platforms that accelerate others' efforts. Some established players are:
- Cortechslabs - focuses on quantitative analysis of brain images. Of particular note is the software called Neuroquant which uses deep learning to produce reports with MRI-based volumetric measurements of structures inside brain that are related to age-related neurodegenerative disorders such as Alzheimer's. Sounds familiar? :)
- Mirada Medical - Oxford-based company that advanced a field of radiation oncology with its deep-learning-based segmentation models
- Arterys - Silicon Valley startup that was the first to obtain an FDA clearance for a deep learning medical imaging suite for oncology.
- Enlitic - San Francisco-based company aiming at diagnostic use cases that accelerate radiologic workflow
- Nuance is a Boston-based company that produces a well established platform of choice for radiological dictation. Recently the company focused a lot of effort on a marketplace for medical imaging AI solutions where startups that do not quite have Nuance's reach can deploy their software.
- Terarecon - similarly to Nuance, this Californian company started in core diagnostic radiology and expanded with an AI marketplace offering branded "EnvoyAI"
Some big cloud providers are eyeing the space closely, and running their own programs and projects related to medical imaging.
- Microsoft Research has a project dubbed InnerEye that for the past 10+ years has been exploring the use of machine learning for a variety of medical imaging applications. One of the instructors of this course had the honor of spending a significant part of his career as a team member here.
- Google DeepMind is a group within Google doing some cutting-edge AI research, including some work on medical imaging. We can credit them with the contribution to the invention of the U-net which has been prominently featured in this course.
TODO: Instructions for how to get a copy of the project running on your local machine.
TODO: Instructions for setting up the environment from Sect3
This project is licensed under the MIT License - see the LICENSE.md
[1] www.sciencedirect.com/science/article/pii/S2213158219302542
[2] en.wikipedia.org/wiki/Hippocampus
[3] medicaldecathlon.com/