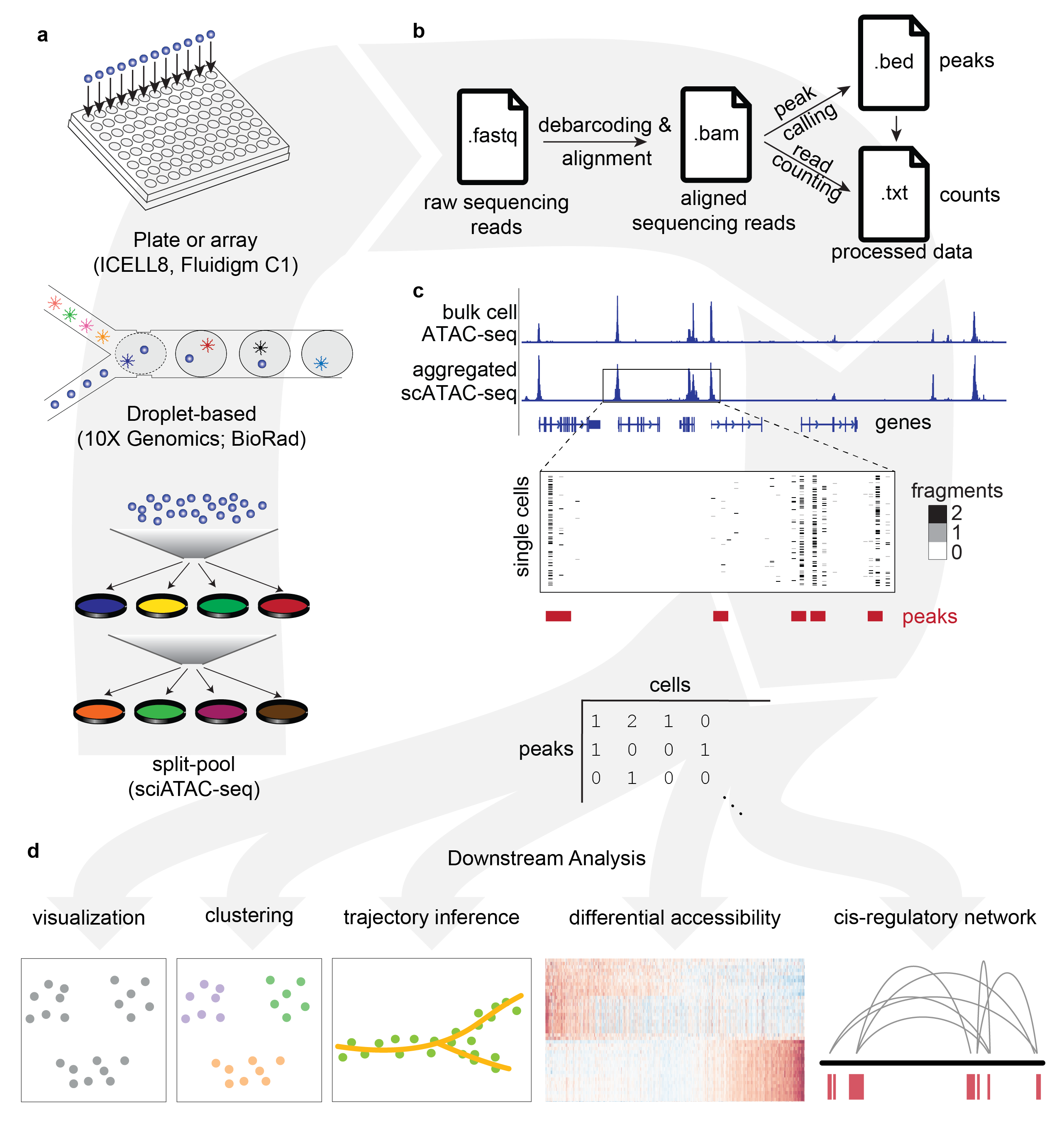
Recent innovations in single-cell Assay for Transposase Accessible Chromatin using sequencing (scATAC-seq) enable profiling of the epigenetic landscape of thousands of individual cells. scATAC-seq data analysis presents unique methodological challenges. scATAC-seq experiments sample DNA, which, due to low copy numbers (diploid in humans) lead to inherent data sparsity (1-10% of peaks detected per cell) compared to transcriptomic (scRNA-seq) data (20-50% of expressed genes detected per cell). Such challenges in data generation emphasize the need for informative features to assess cell heterogeneity at the chromatin level.
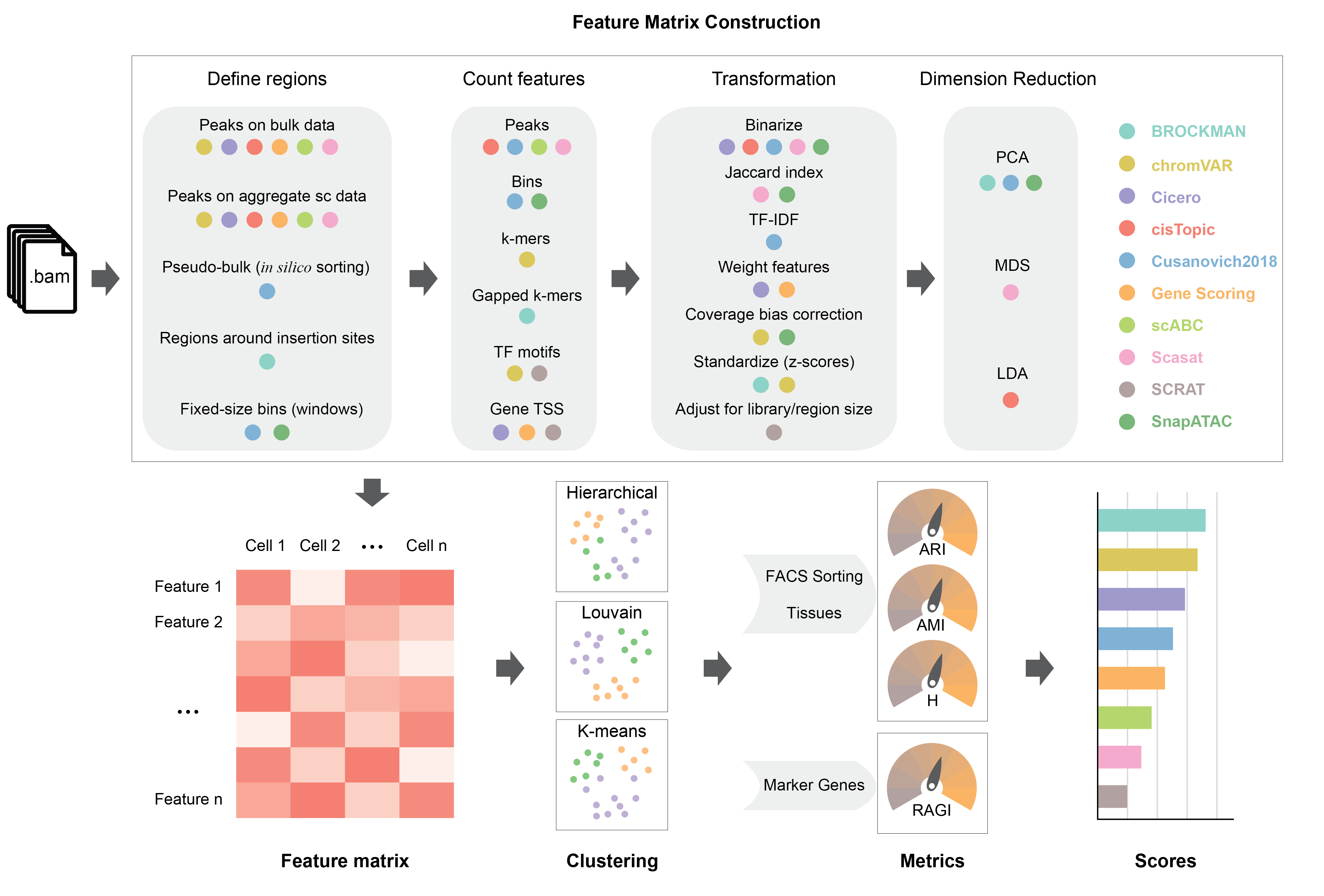
We present a benchmarking framework that was applied to 10 computational methods for scATAC-seq on 13 synthetic and real datasets from different assays, profiling cell types from diverse tissues and organisms. Methods for processing and featurizing scATAC-seq data were evaluated by their ability to discriminate cell types when combined with common unsupervised clustering approaches. We rank evaluated methods and discuss computational challenges associated with scATAC-seq analysis including inherently sparse data, determination of features, peak calling, the effects of sequencing coverage and noise, and clustering performance. Running times and memory requirements are also discussed.
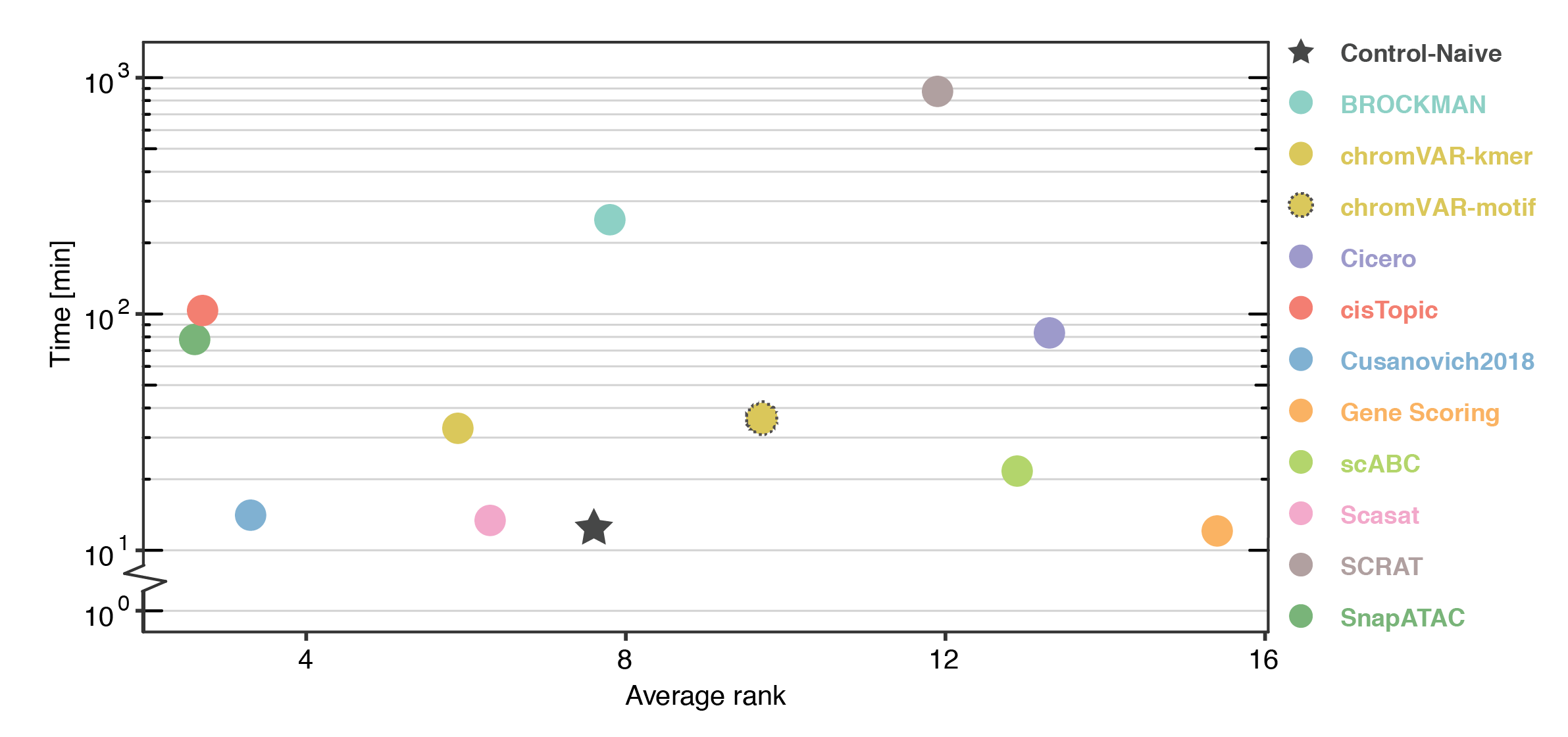
Our benchmarking results highlight SnapATAC, cisTopic, and Cusanovich2018 as the top performing scATAC-seq data analysis methods to perform clustering across all datasets and different metrics. Methods that preserve information at the peak-level (cisTopic, Cusanovich2018, Scasat) or bin-level (SnapATAC) generally outperform those that summarize accessible chromatin regions at the motif/k-mer level (chromVAR, BROCKMAN, SCRAT) or over the gene-body (Cicero, Gene Scoring). In addition, methods that implement a dimensionality reduction step (BROCKMAN, cisTopic, Cusanovich2018, Scasat, SnapATAC) generally show advantages over the other methods without this important step. SnapATAC is the most scalable method; it was the only method capable of processing more than 80,000 cells. Cusanovich2018 is the method that best balances analysis performance and running time.
All the analyses performed are illustrated in Jupyter Notebooks.
Within each dataset folder, the folder 'output' stores all the output files and it consists of five sub-folders including 'feature_matrices', 'umap_rds', 'clusters', 'metrics', and 'figures'.
-
The Buenrostro2018 dataset (folder 'Buenrostro_2018')
- input
- run_methods
- clustering evaluation
- UMAP visualization
- end-to-end clustering (folder 'extra_clustering')
- end-to-end clustering evaluation
- output
-
The Buenrostro2018 using bulk peaks dataset (folder 'Buenrostro_2018_bulkpeaks')
-
The 10X PBMCs dataset (folder '10x_PBMC_5k')
- input
- run_methods
- clustering evaluation
- UMAP visualization
- end-to-end clustering (folder 'extra_clustering')
- end-to-end clustering evaluation
- output
-
The downsampled sci-ATAC-seq-mouse dataset (folder 'Cusanovich_2018_subset')
- input
- run_methods
- clustering evaluation
- UMAP visualization
- end-to-end clustering (folder 'extra_clustering')
- end-to-end clustering evaluation
- output
-
The full sci-ATAC-seq-mouse dataset (folder 'Cusanovich_2018')
- input
- run_methods
- clustering evaluation
- UMAP visualization
- output
-
Data Simulation (folder 'Simulate_scATAC')
-
Bone Marrow
-
Erythropoiesis
-
-
The simulated bone marrow (noise level 0.0) dataset (folder 'BoneMarrow_clean')
-
The simulated bone marrow (noise level 0.2) dataset (folder 'BoneMarrow_noisy_p2')
-
The simulated bone marrow (noise level 0.4) dataset (folder 'BoneMarrow_noisy_p4')
-
The simulated bone marrow (coverage 5k reads) dataset (folder 'BoneMarrow_cov5000')
-
The simulated bone marrow (coverage 2.5k reads) dataset (folder 'BoneMarrow_cov2500')
-
The simulated bone marrow (coverage 1k reads) dataset (folder 'BoneMarrow_cov1000')
-
The simulated bone marrow (coverage 500 reads) dataset (folder 'BoneMarrow_cov500')
-
The simulated bone marrow (coverage 250 reads) dataset (folder 'BoneMarrow_cov250')
-
The simulated erythropoiesis (noise level 0.0) dataset (folder 'Erythropoiesis_clean')
-
The simulated erythropoiesis (noise level 0.2) dataset (folder 'Erythropoiesis_noisy_p2')
-
The simulated erythropoiesis (noise level 0.4) dataset (folder 'Erythropoiesis_noisy_p4')
-
Test first PC
-
Test peaks
- frequency-based peak selection
- intensity-based peak selection
-
Test pseudo-bulk
-
Test blacklist
-
Test parameter settings
Citation: Please cite our preprint if you find this benchmarking work is helpful to your research. Chen, H. et al. Assessment of computational methods for the analysis of single-cell ATAC-seq data. bioRxiv 739011v1 (Aug 18, 2019).
Credits: H Chen, C Lareau, T Andreani, ME Vinyard, SP Garcia, K Clement, MA Andrade-Navarro, JD Buenrostro, L Pinello