Cytometry by time-of-flight(CyTOF) data is very useful in studying the presence/absence of antigens/surface markers at single cell level. There are multiple tools to analyze CyTOF data but here I am presenting a tutorial of how one can quickly use Seurat (R package for scRNA-Seq analysis) [https://satijalab.org/seurat/] for analyzing CyTOF data and understanding the cellular and phenotypic diversity at cellular level.
Step1: Reading .fcs files using read.flowSet function from flowCore R package
Step2: Using Arcsinh transformation to normalize the fcs files. Read more [https://support.cytobank.org/hc/en-us/articles/206148057-About-the-Arcsinh-transform]
Step3: Create a Seurat Object using normalized counts from .fcs files
Step4: Run Dimensionality reduction, clustering and then visualize cells
Step5: Integrate Seurat objects to understand similarities and differences among cell-types learnt from each .fcs file
readxl: https://readxl.tidyverse.org/
Let's read the .fcs files using read.flowSet. [Here I am showing analysis tutorial for 2 fcs samples]
fcs_raw1 <- read.flowSet('fcs_raw1.fcs', path = getwd(), transformation = FALSE, truncate_max_range = FALSE)
fcs_raw2 <- read.flowSet('fcs_raw2.fcs', path = getwd(), transformation = FALSE, truncate_max_range = FALSE)
panel_filename <- "CyTOF_panel.xlsx"
panel <- read_excel(panel_filename)
panel$Antigen <- gsub("/", "_", panel$Antigen)
panel$Antigen <- gsub("-", "_", panel$Antigen)
panel_fcs_raw1 <- pData(parameters(fcs_raw1[[1]]))
panel_fcs_raw2 <- pData(parameters(fcs_raw2[[1]]))
panel_fcs_raw1$desc <- gsub("/", "_", panel_fcs_raw1$desc)
panel_fcs_raw1$desc <- gsub("-", "_", panel_fcs_raw1$desc)
panel_fcs_raw2$desc <- gsub("/", "_", panel_fcs_raw2$desc)
panel_fcs_raw2$desc <- gsub("-", "_", panel_fcs_raw2$desc)
(lineage_markers <- panel$Antigen[panel$Lineage == 1])
(functional_markers <- panel$Antigen[panel$SurfaceMarkers == 1])
fcs_1 <- fsApply(fcs_raw1, function(x, cofactor = 5){
colnames(x) <- panel_fcs_raw1$desc
expr <- exprs(x)
expr <- asinh(expr[, c(panel_fcs_raw1$desc)] / cofactor)
exprs(x) <- expr
x
})
fcs_1
fcs_2 <- fsApply(fcs_raw2, function(x, cofactor = 5){
colnames(x) <- panel_fcs_raw2$desc
expr <- exprs(x)
expr <- asinh(expr[, c(panel_fcs_raw2$desc)] / cofactor)
exprs(x) <- expr
x
})
fcs_2
expr_fcs_1 <- fsApply(fcs_1, exprs)
expr_fcs_1 <- as.matrix(t(expr_fcs_1))
Cells <- c()
SampleName <- c()
TimePoint <- c()
for (f in 1:ncol(expr_fcs_1))
{
a <- paste("sample_",f, sep="")
Cells <- c(Cells,a)
a <- paste("sample")
SampleName <- c(SampleName, a)
a <- paste("time")
TimePoint <- c(TimePoint, a)
}
colnames(expr_fcs_1) <- Cells
metadata <- cbind(Cells, SampleName, TimePoint)
metadata <- data.frame(metadata)
pd <- new("AnnotatedDataFrame", data = metadata)
rownames(pd) <- pd$Cells
Obj_fcs_1 <- CreateSeuratObject(expr_fcs_1)
CellsMeta = Obj_fcs_1@meta.data
CellsMeta["SampleName"] <- pd$SampleName
CellsMetaTrim <- subset(CellsMeta, select = c("SampleName"))
Obj_fcs_1 <- AddMetaData(Obj_fcs_1, CellsMetaTrim)
CellsMeta = Obj_fcs_1@meta.data
CellsMeta["TimePoint"] <- pd$TimePoint
CellsMetaTrim <- subset(CellsMeta, select = c("TimePoint"))
Obj_fcs_1 <- AddMetaData(Obj_fcs_1, CellsMetaTrim)
expr_fcs_2 <- fsApply(fcs_2, exprs)
expr_fcs_2 <- as.matrix(t(expr_fcs_2))
Cells <- c()
SampleName <- c()
TimePoint <- c()
for (f in 1:ncol(expr_fcs_2))
{
a <- paste("sample_",f, sep="")
Cells <- c(Cells,a)
a <- paste("sample")
SampleName <- c(SampleName, a)
a <- paste("time")
TimePoint <- c(TimePoint, a)
}
colnames(expr_fcs_2) <- Cells
metadata <- cbind(Cells, SampleName, TimePoint)
metadata <- data.frame(metadata)
pd <- new("AnnotatedDataFrame", data = metadata)
rownames(pd) <- pd$Cells
Obj_fcs_2 <- CreateSeuratObject(expr_fcs_2)
CellsMeta = Obj_fcs_2@meta.data
CellsMeta["SampleName"] <- pd$SampleName
CellsMetaTrim <- subset(CellsMeta, select = c("SampleName"))
Obj_fcs_2 <- AddMetaData(Obj_fcs_2, CellsMetaTrim)
CellsMeta = Obj_fcs_2@meta.data
CellsMeta["TimePoint"] <- pd$TimePoint
CellsMetaTrim <- subset(CellsMeta, select = c("TimePoint"))
Obj_fcs_2 <- AddMetaData(Obj_fcs_2, CellsMetaTrim)
VariableFeatures(Obj_fcs_1) <- rownames(Obj_fcs_1)
Obj_fcs_1 <- ScaleData(Obj_fcs_1)
Obj_fcs_1 <- RunPCA(Obj_fcs_1, verbose = TRUE)
Obj_fcs_1 <- FindNeighbors(Obj_fcs_1, dims = 1:10, verbose = TRUE)
Obj_fcs_1 <- FindClusters(Obj_fcs_1, verbose = TRUE)
Obj_fcs_1 <- RunUMAP(Obj_fcs_1, dims = 1:10, verbose = TRUE)
Obj_fcs_1 <- RunTSNE(Obj_fcs_1, dims = 1:10, verbose = TRUE)
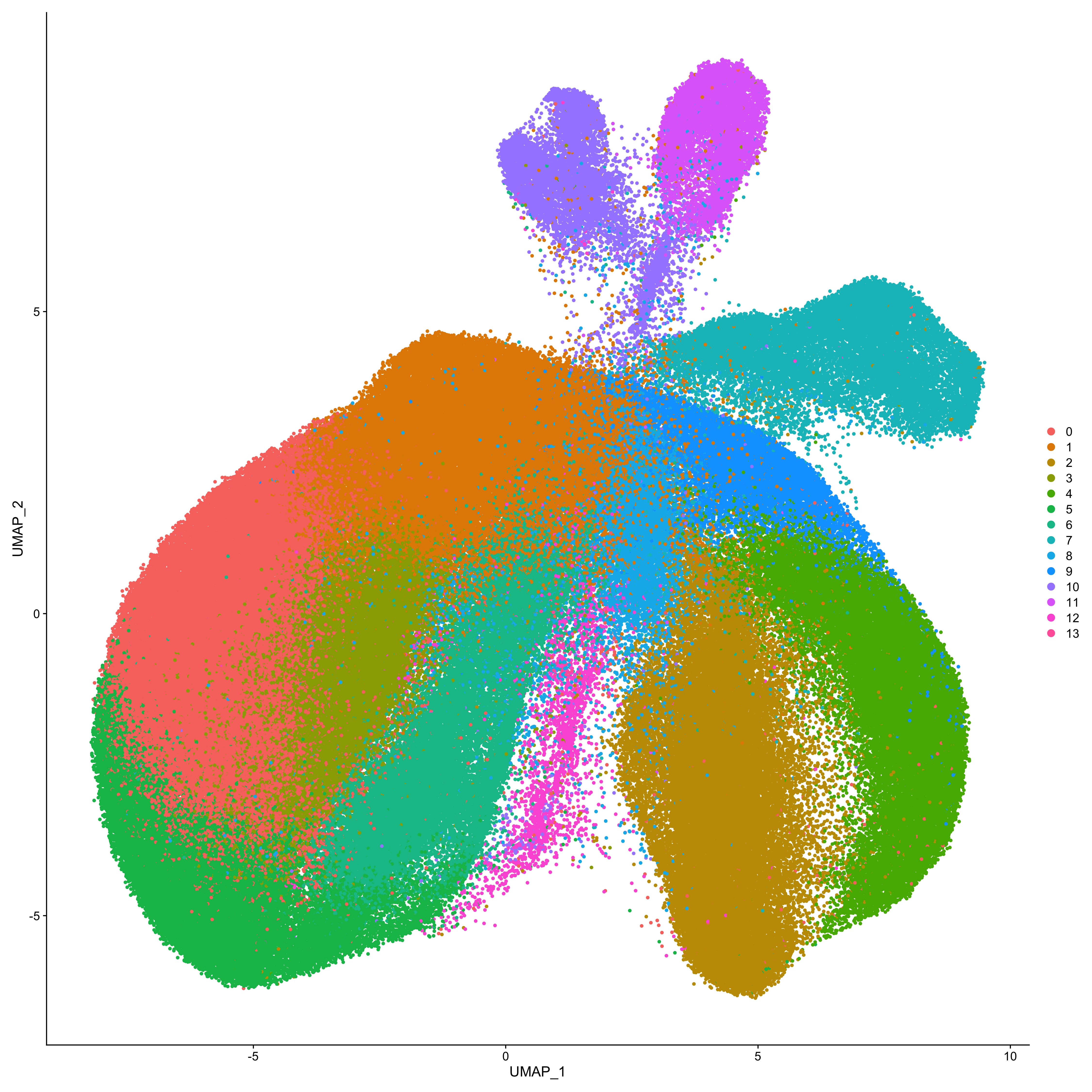
DimPlot(Obj_fcs_1, reduction="umap", pt.size=1)
DimPlot(Obj_fcs_1, reduction="tsne", pt.size=1)
VariableFeatures(Obj_fcs_2) <- rownames(Obj_fcs_2)
Obj_fcs_2 <- ScaleData(Obj_fcs_2)
Obj_fcs_2 <- RunPCA(Obj_fcs_2, verbose = TRUE)
Obj_fcs_2 <- FindNeighbors(Obj_fcs_2, dims = 1:10, verbose = TRUE)
Obj_fcs_2 <- FindClusters(Obj_fcs_2, verbose = TRUE)
Obj_fcs_2 <- RunUMAP(Obj_fcs_2, dims = 1:10, verbose = TRUE)
Obj_fcs_2 <- RunTSNE(Obj_fcs_2, dims = 1:10, verbose = TRUE)
DimPlot(Obj_fcs_2, reduction="umap", pt.size=1)
DimPlot(Obj_fcs_2, reduction="tsne", pt.size=1)
Step5: Integration of Obj_fcs_1 and Obj_fcs_2 to uncover similarities and differences among cell types from each sample
Integration.anchors <- FindIntegrationAnchors(object.list = list(Obj_fcs_1, Obj_fcs_2), dims = 1:20)
Integration.combined <- IntegrateData(anchorset = Integration.anchors, dims = 1:20)
DefaultAssay(Integration.combined) <- "integrated"
Integration.combined <- ScaleData(Integration.combined)
Integration.combined <- RunPCA(Integration.combined, verbose = TRUE)
Integration.combined <- RunUMAP(Integration.combined, reduction = "pca", dims = 1:10)
Integration.combined <- RunTSNE(Integration.combined, reduction = "pca", dims = 1:10)
Integration.combined <- FindNeighbors(Integration.combined, reduction = "pca", dims = 1:10)
Integration.combined <- FindClusters(Integration.combined, resolution = 0.5)
DimPlot(Integration.combined, pt.size=1)