Flye is a de novo assembler for single-molecule sequencing reads, such as those produced by PacBio and Oxford Nanopore Technologies. It is designed for a wide range of datasets, from small bacterial projects to large mammalian-scale assemblies. The package represents a complete pipeline: it takes raw PacBio / ONT reads as input and outputs polished contigs. Flye also has a special mode for metagenome assembly.
Currently, Flye will produce collapsed assemblies of diploid genomes, represented by a single mosaic haplotype. To recover two phased haplotypes consider applying HapDup after the assembly.
- Disjointig step speedup for
--nano-hqmode - Improved
--keep-haplotypesmode preserves more heterozygous SVs - A few bug fixes
- Update to minimap 2.24 + using HiFi and Kit14 parameters for faster alignment
- Fixed a few small bugs and corner cases
- Polisher now outputs read depth for each base of consensus (can be used as confidence measure)
- New option --no-alt-contigs to remove all non-primary contigs from the assembly
- Fixed crash on MacOS with Python 3.8+
- Fixed rare artificially introduced mismatches while polishing
- Fixed slow simplification of very tangled graphs
- Various other small fixes
- Better assembly of very short sequences (e.g. plasmids or viruses). They were often missed in previous versions.
- New --nano-hq mode for ONT Guppy5+ (SUP mode) and Q20 reads (3-5% error rate)
- Optimized default parameters for HiFi (HPC error threshold 0.01 -> 0.001; increased min overlap)
- Polishing improvements: reduced number of possible clusters of errors
- Improvements in repeat detection algorithm to further limit a chance of (otherwise infrequent) misassemblies
- Scaffolding is no longer performed by default (could be enabled with --scaffold)
- Bam file input for the standalone polisher (same interface as for FASTA/Q)
- Automatically selected minimum overlap up to 10k (was 5k)
- Discontinued --plasmid option due to the improvements in short sequences assembly
- --trestle and --subassemblies modes are now deprecated, and will be removed in the future versions
- New --extra-params option to modify config-level parameters
- Contig paths output in Gfa + number of reads supporting each link (RC tag)
- Update to minimap 2.18
- Several rare bug fixes/other improvements
Flye is using a repeat graph as the core data structure. Compared to de Bruijn graphs (which require exact k-mer matches), repeat graphs are built using approximate sequence matches, and can tolerate the higher noise of SMS reads.
The edges of a repeat graph represent the genomic sequence, and nodes define the junctions. Each edge is classified as unique or repetitive. The genome traverses the graph (in an unknown way), so each unique edge appears exactly once in this traversal. Repeat graphs reveal the repeat structure of the genome, which helps to reconstruct an optimal assembly.
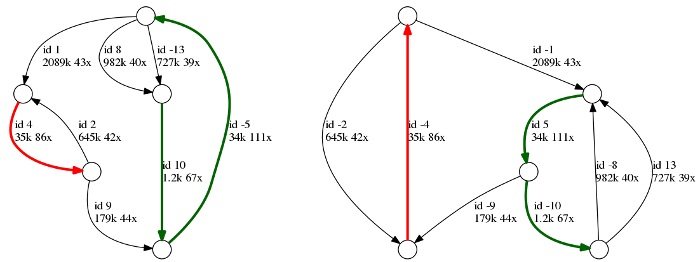
Above is an example of the repeat graph of a bacterial assembly. Each edge is labeled with its id, length, and coverage. Repetitive edges are shown in color, and unique edges are black. Note that each edge is represented in two copies: forward and reverse complement (marked with +/- signs), therefore the entire genome is represented in two copies. This is necessary because the orientation of input reads is unknown.
In this example, there are two unresolved repeats: (i) a red repeat of multiplicity two and length 35k and (ii) a green repeat cluster of multiplicity three and length 34k - 36k. As the repeats remained unresolved, there are no reads in the dataset that cover those repeats in full. Five unique edges will correspond to five contigs in the final assembly.
Repeat graphs produced by Flye could be visualized using AGB or Bandage.
| Genome | Data | Asm.Size | NG50 | CPU time | RAM |
|---|---|---|---|---|---|
| E.coli | PB 50x | 4.6 Mb | 4.6 Mb | 2 h | 2 Gb |
| C.elegans | PB 40x | 107 Mb | 2.7 Mb | 100 h | 31 Gb |
| A.thaliana | PB 75x | 120 Mb | 8.7 Mb | 100 h | 59 Gb |
| D.melanogaster | ONT 30x | 136 Mb | 13.8 Mb | 130 h | 33 Gb |
| D.melanogaster | PB 120x | 141 Mb | 11.5 Mb | 150 h | 70 Gb |
| Human NA12878 | ONT 35x (rel6) | 2.8 Gb | 30.3 Mb | 3100 h | 394 Gb |
| Human CHM13 ONT | ONT 120x (rel5) | 2.9 Gb | 69.5 Mb | 4000 h | 450 Gb |
| Human CHM13 HiFi | PB HiFi 30x | 3.0 Gb | 34.8 Mb | 780 h | 141 Gb |
| Human HG002 | PB ONT 110x | 2.9 Gb | 46.9 Mb | 4000 h | 409 Gb |
| Human CHM1 | PB 100x | 2.8 Gb | 18.6 Mb | 2700 h | 444 Gb |
| Cliveome Q20 | ONT 35x | 3.0 Gb | 46.5 Mb | 2000 h | 257 Gb |
| HMP mock | PB meta 7 Gb | 68 Mb | N/A | 60 h | 72 Gb |
| Zymo Even | ONT meta 14 Gb | 65 Mb | N/A | 60 h | 129 Gb |
| Zymo Log | ONT meta 16 Gb | 29 Mb | N/A | 100 h | 76 Gb |
| Sheep gut | HiFi meta 255G | 4.2 Gb | N/A | 3500 h | 662 Gb |
The assemblies generated using Flye 2.9 could be downloaded from Zenodo.
All datasets were run with default parameters for the corresponding read type
with the following exceptions: CHM13 T2T, CHM1 and HG002 were run with --asm-coverage 50.
Note that this version of the table reflects contigs NG50, while the previous versions were referring to scaffold NG50.
Flye package includes some third-party software:
Flye is distributed under a BSD license. See the LICENSE file for details.
Flye is developed in Pavel Pevzner's lab at UCSD
Main code contributors:
- metaFlye: Mikhail Kolmogorov
- Repeat graph and current package maintaining: Mikhail Kolmogorov
- Trestle module and original polisher code: Jeffrey Yuan
- Original contig extension code: Yu Lin
- Short plasmids recovery module: Evgeny Polevikov
Mikhail Kolmogorov, Derek M. Bickhart, Bahar Behsaz, Alexey Gurevich, Mikhail Rayko, Sung Bong Shin, Kristen Kuhn, Jeffrey Yuan, Evgeny Polevikov, Timothy P. L. Smith and Pavel A. Pevzner "metaFlye: scalable long-read metagenome assembly using repeat graphs", Nature Methods, 2020 doi:s41592-020-00971-x
Mikhail Kolmogorov, Jeffrey Yuan, Yu Lin and Pavel Pevzner, "Assembly of Long Error-Prone Reads Using Repeat Graphs", Nature Biotechnology, 2019 doi:10.1038/s41587-019-0072-8
Yu Lin, Jeffrey Yuan, Mikhail Kolmogorov, Max W Shen, Mark Chaisson and Pavel Pevzner, "Assembly of Long Error-Prone Reads Using de Bruijn Graphs", PNAS, 2016 doi:10.1073/pnas.1604560113
How to cite: the 2020 paper is the most relevant to metagenome assembly. For single genome assembly, use the 2019 paper as reference. The 2016 paper describes solid k-mer indexing and polishing approaches that are used as core algorithms in the current pipeline.
A preferred way report any problems or ask questions about Flye is the issue tracker. Before posting an issue/question, consider to look through the FAQ and existing issues (opened and closed) - it is possible that your question has already been answered.
If you are reporting a problem, please include the flye.log file and provide
details about your dataset.
In case you prefer personal communication, please contact Mikhail at mikolmogorov@gmail.com.