Additional code, analyses and results to extend the supplementary information of:
Fellows Yates, J. A. et al. (2021) ‘The evolution and changing ecology of the African hominid oral microbiome’, Proceedings of the National Academy of Sciences of the United States of America, 118(20), p. e2021655118. doi: 10.1073/pnas.2021655118.
Please read section R1 before proceeding.
- Evolution of the Hominid Calculus Microbiome
- Table of Contents
- R1 Introduction
- R2 Resources
- R3 Software Paths
- R4 Database and Genome Indexing
- R5 Data Acquisition
- R6 Data Preprocessing
- R7 Metagenomic Screening
- R8 Preservation Screening
- R9 Compositional Analysis
- R10 Core Microbiome Analysis
- R11 Genome Reconstruction and Phylogenetics
- R11.1 Production dataset sequencing depth calculations
- R11.2 Super-reference construction
- R11.3 Super-reference alignment and species selection
- R11.4 Comparative single reference mapping
- R11.5 Performance of super-reference vs. single genome mapping
- R11.6 Variant calling and single-allelic position assessment
- R11.7 Phylogenies
- R11.8 Pre- and Post-14k BP Observation Verification
- R12 Functional Analysis
This README acts as walkthrough guidance of the order of analyses for Fellows Yates, J.A. et al. (2021) PNAS. It acts as a practical methods supplement as well as for displaying additional figures only. Justification and discussion of figures and results are sparsely described, and the main publication should be referred to for scientific interpretation and context.
The repository contains additional data files, R notebooks, scripts and commands where a program was initiated directly from the command line, as well as some additional results.
The following objects that are referred to in the supplementary information of the paper can be found here:
- External Data Repository Section RXX (in this README)
- External Data Repository Figure RXX (in this README and under
05-images/.) - External Data Repository Table RXX (in this README)
- External Data Repository File RXX (in this README and under
06-additional_data_files/.)
Code can be found under the directory 02-scripts.backup, 'raw'
analysis results can be seen under 04-analysis. 'Cleaned-up'/summary analysis
results and metadata files can be seen under 00-documentation. Figures
displayed within this README are stored under 05-images. Additional data
files for fast access/referral in the main publication can be seen under
06-additional_data_files.
01-dataand03-preprocessingonly contains empty directories as these contain very large sequencing files that cannot be uploaded to this repository. They are kept here so if reproducing analysis, paths in scripts do not need to be modified. Raw data can be found on the ENA under project accession ID: PRJEB34569.
In more detail, the general structure of this project is typically as follows (although variants will occur):
README.md ## This walkthrough
00-documentation/ ## Contains main metadata files and summary result files
00-document_1.txt
01-document_2.txt
01-data/ ## Only contains directories where raw data FASTQ files
raw_data/ ## are stored (must be downloaded yourself, not included
screening/ ## due to large file size)
deep/
databases/
<database_1>/
02-scripts.backup/ ## Contains all scripts and R notebooks used in this
000-ANALYSIS_CONFIG ## project and referred to throughout this README
001-script.sh
002-notebook.Rmd
03-preprocessing ## Only contains directories where pre-processed FASTQ
screening/ ## files are stored (must be processed yourself, not
human_filtering/ ## included due to large file size)
input/
output/
library_merging/
input/
output/
deep/
human_filtering
input/
output/
library_merging/
input/
output/
04-analysis/ ## Contains all small(ish) results files from analyses,
screening/ ## such as tables, .txt. files etc. Large files (BAM, RAM6
analysis_1/ ## etc.) not included here due to large suze and must be
input ## generated yourself
output
analysis_2/
input
output
deep/
analysis_1/
input
output
analysis_2/
input
output
05-images/ ## Stores images displayed in this README
Figure_R01/
Figure_R02/
06-additional_data_files ## A fast look-up location for certain important files
## referred to in the supplementary text of the
## associated manuscript. Typically copies of files
## stored in 04-analysis.
Important: The code in this repository was written over multiple 'learning' years by non-bioinformaticians. Quality will vary and may not be immediately re-runable or readable - if you encounter any issues please leave an issue and we will endeavour to clarify.
We have tried to auto-replace all file paths to make it relative to this repository. This may not have been perfect, so please check the path begins with
../0{1,2,4}. If it does not, let us know and we will fix this accordingly.
Here is a list of programs and databases that will be used in this analysis and that you should have ready installed/downloaded prior carrying out the analysis. Note that the download and set up of the databases are described below.
This analysis was performed on a server running Ubuntu 14.04 LTS, and a SLURM submission scheduler. Some scripts or commands may heavily refer to SLURM, however these are directly related to our system. As far as we can, we have removed SLURM commands and/or MPI-SHH specific paths or parameters, but you should always check this for each command and script.
| Name | Version | Citation |
|---|---|---|
| GNU bash | 4.3.11 | NA |
| sratoolkit | 2.8.0 | https://www.ncbi.nlm.nih.gov/books/NBK158900/ |
| EAGER | 1.9.55 | Peltzer et al. 2016 Genome Biol. |
| AdapterRemoval | 2.3.0 | Schubert et al. 2016 BMC Research Notes |
| bwa | 0.7.12 | Li and Durbin 2009 Bioinformatics |
| samtools | 1.3 | Li et al. 2009 Bioinformatics |
| PicardTools | 1.140 | https://github.com/broadinstitute/picard |
| GATK | 3.5 | DePristo et al. 2011 Nat. Genets. |
| mapDamage | 2.0.6 | Jónsson et al. 2013 Bioinformatics |
| DeDup | 0.12.1 | Peltzer et al. 2016 Genome Bio |
| MALT | 0.4.0 | Herbig et al. 2016 bioRxiv, Vagene et al. 2018 Nat. Eco. Evo. |
| MEGAN CE | 6.12.0 | Huson et al. 2016 PLoS Comp. Bio. |
| FastP | 0.19.3 | Chen et al. 2018 Bioinformatics |
| Entrez Direct | Jun 2016 | http://www.ncbi.nlm.nih.gov/books/NBK179288 |
| QIIME | 1.9.1 | Caporaso et al. 2010 Nat. Methods. |
| Sourcetracker | 1.0.1 | Knights et al. 2011 Nat. Methods. |
| conda | 4.7.0 | https://conda.io/projects/conda/en/latest/ |
| pigz | 2.3 | https://zlib.net/pigz/ |
| R | >=3.5 | https://www.R-project.org/ |
| MaltExtract | 1.5 | Hübler et al. 2019 Genome Biology |
| MultiVCFAnalyzer | 0.87 | Bos et al. 2014 Nature |
| bedtools | 2.25.0 | Quinlan et al. 2010 Bioinformatics |
| panX | 1.5.1 | Ding et al. 2018 Nucleic Acids Research |
| MetaPhlAn2 | 2.7.1 | Truong et al. 2015 Nat. Methods |
| HuMAnN2 | 0.11.2 | Franzosa et al. 2018 Nat. Methods. |
| blastn | 2.7.1+ | Package: blast 2.7.1, build Oct 18 2017 19:57:24 |
| seqtk | 1.2-r95-dirty | https://github.com/lh3/seqtk |
| Geneious | R8 | https://www.geneious.com/ |
| IGV | 2.4 | https://software.broadinstitute.org/software/igv/ |
| Inkscape | 0.92 | www.inkscape.org |
Here we used R version 3.6.1
If did not come with package itself, we downloaded the following:
| Name | Version | Date | Download Location |
|---|---|---|---|
| NCBI Nucleotide | nt.gz | Dec. 2016 | ftp://ftp.ncbi.nlm.nih.gov/blast/db/FASTA/ |
| NCBI RefSeq | Custom | Nov. 2018 | ftp://ftp.ncbi.nlm.nih.gov/genomes/ |
| SILVA | 128_SSURef_Nr99 | Mar. 2017 | http://ftp.arb-silva.de/release_128/Exports/ |
| UniRef | uniref90_ec_filtered_diamond | Oct. 2018 | https://bitbucket.org/biobakery/humann2 |
⚠️ SILVA FASTA was modified after downloading replacing Us with Cs
| Species | Strain | Date | Completeness | Type | Source |
|---|---|---|---|---|---|
| Homo sapiens | HG19 | 2016-01-14 | Complete | Reference | http://hgdownload.cse.ucsc.edu/downloads.html#human |
| Actinomyces dentalis | DSM 19115 | 2019-02-25 | Scaffold | Representative | ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/000/429/225/GCF_000429225.1_ASM42922v1/ |
| Aggregatibacter aphrophilus | W10433 | 2017-06-14 | Complete | Representative | ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/000/022/985/GCF_000022985.1_ASM2298v1/ |
| Campylobacter gracilis | - | 2019-05-22 | Complete | Representative | ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/001/190/745/GCF_001190745.1_ASM119074v1/ |
| Capnocytophaga gingivalis | ATCC 33624 | 2019-02-25 | Contigs | Representative | ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/000/174/755/GCF_000174755.1_ASM17475v1/ |
| Corynebacterium matruchotii | ATCC 14266 | 2019-05-22 | Contigs | Representative | ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/000/175/375/GCF_000175375.1_ASM17537v1/ |
| Desulfobulbus sp. oral taxon 041 | Dsb1-5 | 2018-06-06 | Contigs | Assembly | ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCA/000/403/865/GCA_000403865.1_Dsb1-5/ |
| Fretibacterium fastidiosum | - | 2018-12-11 | Chromosome | Unknown | https://www.ncbi.nlm.nih.gov/nuccore/FP929056.1 |
| Fusobacterium hwasookii | ChDC F206 | 2019-10-27 | Complete | Representative | ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/001/455/105/GCF_001455105.1_ASM145510v1 |
| Fusobacterium nucleatum subsp. nucleatum | ATCC 25586 | 2017-06-14 | Complete | Representative | ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/000/007/325/GCF_000007325.1_ASM732v1/ |
| Olsenella sp. oral taxon 807 | 807 | 2019-01-10 | Complete | Unknown | ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/001/189/515/GCF_001189515.2_ASM118951v2/ |
| Ottowia sp. oral taxon 894 | 894 | 2019-05-22 | Complete | Unknown | ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/001/262/075/GCF_001262075.1_ASM126207v1/ |
| Porphyromonas gingivalis | ATCC 33277 | 2018-12-11 | Complete | Representative | ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/000/010/505/GCF_000010505.1_ASM1050v1/ |
| Prevotella loescheii | DSM 19665 | 2019-05-22 | Scaffolds | Representative | ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/000/378/085/GCF_000378085.1_ASM37808v1/ |
| Pseudopropionibacterium propionicum | F0230a | 2018-12-11 | Complete | Representative | ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/000/277/715/GCF_000277715.1_ASM27771v1 |
| Rothia dentocariosa | ATCC 17831 | 2017-06-14 | Complete | Representative | ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/000/164/695/GCF_000164695.2_ASM16469v2/ |
| Selenomonas sp. F0473 | F0473 | 2019-05-22 | Scaffolds | Unknown | ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/000/315/545/GCF_000315545.1_Seleno_sp_F0473_V1/ |
| Streptococcus gordonii | CH1 | 2018-06-14 | Complete | Representative | ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/000/017/005/GCF_000017005.1_ASM1700v1 |
| Streptococcus sanguinis | SK36 | 2018-12-11 | Complete | Reference | ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/000/014/205/GCF_000014205.1_ASM1420v1/ |
| Tannerella forsythia | 92A2 | 2018-12-18 | Complete | Representative | ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/000/238/215/GCF_000238215.1_ASM23821v1/ |
| Treponema socranskii subsp. paredies | ATCC 35535 | 2018-05-31 | Scaffolds | Representative | ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/000/413/015/GCF_000413015.1_Trep_socr_subsp_paredis_ATCC_35535_V1/ |
⚠️ The reference files are not provided here due to their large size.
Some scripts used in this project use variables stored a central profile called
02-scripts.backup/000-analysis_profile. These variables indicate the location
of certain programs in your servers file-system. The scripts loading the
profile will therefore look for and use the program stored in the variable.
You will need to replace the paths stored there to where each tool is stored
on your personal server, I have replaced our central storage to <YOUR_PATH>,
however you will need to check each path correctly.
Note: not all scripts use the 'profile', so please check each one before running
For direct commands (i.e. not used in a script), the path will either
be defined in the command block or assumed already in your $PATH.
The MALT nt databases was downloaded and generated as follows.
## MALT indexed NT database
mkdir -p 01-data/databases/malt/raw 01-data/databases/malt/indexed
cd 01-data/databases/malt/raw
### Download nucleotide database fasta and md5sum file into a database directory
wget ftp://ftp-trace.ncbi.nih.gov/blast/db/FASTA/nt.gz .
wget ftp://ftp-trace.ncbi.nih.gov/blast/db/FASTA/nt.gz.md5 .
### Generate the md5sum of the downloaded file, and comapre with contents of
### the NCBI .md5 version
md5sum nt.gz
cat nt.gz.md5
### Download into a different directory the accession to taxonomy mapping file
### as provided on the MEGAN6 website, and unzip
mkdir 01-data/databases/malt/acc2bin
cd !$
wget http://ab.inf.uni-tuebingen.de/data/software/megan6/download/nucl_acc2tax-May2017.abin.zip
unzip nucl_acc2tax-May2017.abin.zip
malt-build \
--step 2 \
-i "$DBDIR"/malt/raw/nt.gz \
-s DNA \
-d "$DBDIR"/malt/indexed \
-t 112 -a2taxonomy "$DBDIR"/malt/raw/nucl_acc2tax-May2017.abin
⚠️ The database files are not provided here due to their large size.
For the custom NCBI Genome RefSeq database containing bacterial and archaea
assemblies at scaffold, chromosome and complete levels - we follow the
R notebook here: 02-scripts.backup/099-refseq_genomes_bacteria_archaea_homo_complete_chromosome_scaffold_walkthrough_20181029.Rmd.
A corresponding list of genomes selected for use in this database can be seen
in in 06-additional_data_files under Data R10.
To build the aadder database for functional analysis - based on the custom
RefSeq MALT database above - we run the command
01-data/027-aadder_build_refseqCGS_bacarch_sbatch_script_20181104.sh. This
calls the adder-build command as provided in the MEGAN install directory's
tools folder. Note we have to change the MEGAN.vmoptions to have a large
enough memory allocation in the MEAN install directory.
The SILVA reference database, and all single genomes (HG19, bacterial etc.) were indexed as follows - with the SILVA database FASTA file as an example.
BWA=<PATH_TO>/bwa
SAMTOOLS=<PATH_TO>/samtools
PICARDTOOLS=<PATH_TO>/picard
mkdir $DBDIR/silva
cd !$
wget http://ftp.arb-silva.de/release_128/Exports/SILVA_128_SSURef_Nr99_tax_silva_trunc.fasta.gz
"$BWA" index SILVA_128_SSURef_Nr99_tax_silva_trunc.fasta
"$SAMTOOLS" faidx SILVA_128_SSURef_Nr99_tax_silva_trunc.fasta
"$PICARDTOOLS" CreateSequenceDictionary \
REFERENCE=SILVA_128_SSURef_Nr99_tax_silva_trunc.fasta \
O=SILVA_128_SSURef_Nr99_tax_silva_trunc.fasta.dict
⚠️ The indexing files are not provided here due to their large size.
For acquiring the UniRef database for HUMANn2, we used the script that comes with HUMANn2, and run as follows:
humann2_databases --download uniref uniref90_ec_filtered_diamond 01-data/databases/uniref90
⚠️ The database files are not provided here due to their large size.
For the scripts using analysis_profile file, ensure to update the paths in
02-scripts.backup/000-analysis_profile to in the analysis profile to your
corresponding location.
## MALT DB Directory containing all database files
MALTDB=<PATH_TO>/malt/databases/indexed/index038/full-nt_2017-10
## SILVA DB directory containing the converted U to T FASTA file and associated bwa indexed files
SILVADB=<PATH_TO>01/databases/silva/release_128_DNA/
## GreenGenes DB directory, as provided in QIIME
GREENGENESDB=<PATH_TO>/tools/qiime-environment/1.9.1/lib/python2.7/site-packages/qiime_default_reference/gg_13_8_otus
HG19REF=<PATH_TO>/Reference_Genomes/Human/HG19/hg19_complete.fastaAll raw FASTQ files should be downloaded to sample specific directories in 01-data/public_data/raw.
General laboratory and sequencing and meta information about all newly generated
libraries for this study can be seen in
06-additional_data_files/ under Data R01 and Data R02.
The same information but for controls can be seen in Data R03.
⚠️ FASTQ files are not provided here due to their large size. Please see ENA under accession ID: PRJEB34569
In addition to the samples sequenced in this study (or generated by our group, but previously published in the case of JAE), we downloaded the shotgun sequenced individuals from Weyrich et al. 2017 (Nature).
For this, I downloaded the 'processed' files from the OAGR database, as the original data had barcodes, and they already had used AdapterRemoval, as done here.
The list of files and hard links can be seen in the documentation under
00-documentation.backup/99-public_data-Weyrich_Neanderthals.txt.
I downloaded each file with wget, renaming with the file as listed in the
OAGR website, concatenated multi-file samples if required (i.e. ElSidron1) then
renamed to the EAGER standard.
An example:
cd 01-data/public_data/
mkdir ElSidron1
wget https://www.oagr.org.au/api/v1/dataset_file/3086/download
rename s/download/2NoAdapt_ELSIDRON1L7_lTACTG_rCTCGA_R1R2_Collapsed.fastq.gz/ download
wget https://www.oagr.org.au/api/v1/dataset_file/3109/download
rename s/download/2NoAdapt_ELSIDRON1L7_lTACTG_rCTCGA_R1R2_Collapsed_Truncated.fastq.gz/ download
cat *fastq.gz > ElSidron1_S0_L000_R1_000.fastq.merged.fq.gz
## If no concatenation required
# mv 2NoAdapt_CHIMP_150519_lACGTG_rATTGA_R1R2_Collapsed.fastq.gz Chimp.150519_S0_L000_R1_000.fastq.merged.fq.gz
The final merged individual fastq files were moved to individual directories in
/01-data/public_data/prepped.
The just renamed files were then symlinked into the folder above.
⚠️ FASTQ files are not provided here due to their large size. Please see corresponding locations described above
In addition to ancient and calculus samples, we also require comparative data from different sources of microbiomes (e.g. soil, skin, gut etc.).
Note that the bone 'environmental' sample comparative samples were previously published, but re-sequenced for this study. The new sequencing files used in this study can also be found in the ENA repository PRJEB34569 alongside all new calculus data.
Comparative source files were selected based on the following criteria:
- Had to be shotgun metagenomes
- Have had no modification or treatment made to DNA selection or host (e.g. no pesticide, as far as could be determined)
- Must have been generated on an Illumina platform
- Must have more than 10 million reads
- Must have yield than 1000 16S rRNA reads in the shotgun data (detected during analysis - see QIIME section)
In addition, the Human Microbiome Project gut and plaque samples had the additional criteria of:
- Each must be from unique individuals
- Aim for approximately 50/50 male and female where possible
The files were downloaded from the NCBI SRA and EBI ENA
databases. A list of these libraries can be seen either in
06-additional_data_files under R04, or in the 'mapping' file
00-documentation.backup/02-microbiome_calculus-deep_evolution-individualscontrolssources_metadata.tsv.
Metadata on the HMP samples can be seen in
00-documentation/99-sourcemetadata-hmp_SraRunTable_allRuns.tsv, with
samples selected for each source type coming from unique individuals, as
inferred by the hmp_subject_id column. Metadata for the other datasets can be
seen in the 00-documentation/99-sourcemetadata-* files
A file containing the ERR and SRR numbers of each library on each line was
given to the scripts 001-SRA_download_script.sh and 002-ERR_download_script.sh.
Some HMP project samples were not available directly from the SRA FTP server,
in which case these were directly pulled using either prefetch -v, which is
a similar command as in the 001-SRA_download_script.sh file, but without
the wget step.
SRATOOLKIT=/projects1/tools/sratoolkit/2.8.0/sratoolkit.2.8.0-ubuntu64/bin
"$SRATOOKIT"/prefetch -v SRR514306
"$SRATOOKIT"/fastq-dump -F --split-files --readids /projects1/clusterhomes/fellows/ncbi/public/sra/SRR514306.sra --gzip --outdir .These were then renamed using
cd 01-data/public_data/raw/
rename s/_1.fastq.gz/_S0_L001_R1_000.fastq.gz/ */*.fastq.gz
rename s/_2.fastq.gz/_S0_L001_R2_000.fastq.gz/ */*.fastq.gz
The final fastq files were then placed symlinked to individual directories in
01-data/public_data/prepped
For the sediment data from Slon et al. 2017, I also resorted to downloading the FASTQ files directly. However, unfortunately, the uploaded data was actually not 'raw' but the already merged data from the Slon 2017 paper. We downloaded the FASTQ data anyway and did a modified pre-processing.
I did this with the following command, and utilising the file generated from
the parsing script of the two metadata files which is stored here
02-scripts.backup/99-Slon2017_DataFinderScript.R.
cat 00-documentation.backup/99-Slon2017_AccessionsToDownload_2.tsv | while read LINE; do
mkdir 01-data/raw_data/screening/$(echo $LINE | cut -d ';' -f1 | rev | cut -d/ -f 2 | rev) && \
wget $(echo $LINE | cut -d ';' -f1) -P 01-data/raw_data/screening/$(echo $LINE | cut -d ';' -f1 | rev | cut -d/ -f 2 | rev)/ && \
wget $(echo $LINE | cut -d ';' -f2) -P 01-data/raw_data/screening/$(echo $LINE | cut -d ';' -f1 | rev | cut -d/ -f 2 | rev)/ && \
wget $(echo $LINE | cut -d ';' -f3) -P 01-data/raw_data/screening/$(echo $LINE | cut -d ';' -f1 | rev | cut -d/ -f 2 | rev)/ && \
rename s/.fastq.gz/_S0_L001_R1_000.merged.fastq.gz/ \
01-data/raw_data/screening/$(echo $LINE | cut -d ';' -f1 | rev | cut -d/ -f 2 | rev)/$(echo $LINE | cut -d ';' -f1 | rev | cut -d/ -f 2 | rev).fastq.gz && \
rename s/_1.fastq.gz/_S0_L001_R1_000.fastq.gz/ 01-data/raw_data/screening/$(echo $LINE | cut -d ';' -f1 | rev | cut -d/ -f 2 | rev)/*.fastq.gz && \
rename s/_2.fastq.gz/_S0_L001_R2_000.fastq.gz/ 01-data/raw_data/screening/$(echo $LINE | cut -d ';' -f1 | rev | cut -d/ -f 2 | rev)/*.fastq.gz
done
To complete standardisation of this data, we combined singleton files.
cat 00-documentation.backup/99-Slon2017_AccessionsToDownload_2.tsv | while read LINE; do
mkdir 03-preprocessing/screening/human_filtering/input/$(echo $LINE | cut -d ';' -f1 | rev | cut -d/ -f 2 | rev)/ && \
cat 01-data/raw_data/screening/$(echo $LINE | cut -d ';' -f1 | rev | cut -d/ -f 2 | rev)/*.fastq.gz >> \
03-preprocessing/screening/human_filtering/input/$(echo $LINE | cut -d ';' -f1 | rev | cut -d/ -f 2 | rev)/$(echo $LINE | cut -d ';' -f1 | rev | cut -d/ -f 2 | rev)_S0_L001_R1_000.merged.fq.gz
doneThe final merged individual fastq files were moved to individual directories in
01-data/public_data/prepped
⚠️ FASTQ files are not provided here due to their large size. Please see corresponding locations described above
Now downloaded, we started preprocessing by performing a sequencing quality check, merged any of the PE data (as well as trimming low quality bases), removed any DNA that maps to the human genome (but preserving the statistics) and extracted all-non human DNA for downstream processing.
We performed this in two different ways. When this project first started our
local build of EAGER was broken, so I made our own script version(s)
(02-scripts.backup/003-preprocessing_human_filtering.sh, and
02-scripts.backup/004-preprocessing_human_filtering_premerged.sh) utilising
the same commands and tool versions as run by EAGER. Once EAGER was fixed, we
returned to using to this as it was more robust. The only addition to the script
version was samtools fastq to convert unmapped reads (i.e. non-human reads,
used for metagenomic screening) to FASTQ.
The output of both methods of preprocessing was stored in 03-preprocessing/screening/human_filtering.
⚠️ Output FASTQ files are not provided here due to their large size.
Procedure is described in more detail in the next sections.
Below I provide a loop that made sure to run the script(s) on libraries we had not
already run based on what was already present in the output
directory. All we needed was to change the paths in the variables INDIR and
OUTDIR.
If you need to increase the number of cores of memory, you can modify this in the
CPUandMEMvariables in the script itself.
You will need to check if these 'manual' scripts are working correctly manually, as I didn't have time to in-built checks. We verified this by checking all the fields in the output of the statistics script (see here) was filled with numbers.
An example of the loop using with 'premerged' script is as follow (remove the
_premerged section of the script name to run for 'raw' FASTQs)
## Cycle through INDIR corresponding to each type of data (paired/single, hiseq/nextseq, pre-merged etc.)
INDIR=03-preprocessing/screening/human_filtering/input/hiseq_single
OUTDIR=03-preprocessing/screening/human_filtering/output
SCRIPTS=02-scripts.backup
for LIBDIR in "$INDIR"/*/; do
LIBNAME=$(echo "$LIBDIR" | rev | cut -d/ -f2 | rev)
if [ -d "$OUTDIR"/"$LIBNAME" ]; then
printf "\n $LIBNAME already Processed \n\n"
else
mkdir "$OUTDIR"/"$LIBNAME"
$SCRIPTS/03-preprocessing_human_filtering_premerged.sh $OUTDIR/$LIBNAME $LIBDIR $LIBNAME
sleep 1
doneFor the Slon et al. 2017 and Weyrich et al. 2017 datasets, we ran EAGER without AdapterRemoval as the ENA/OAGR uploaded data was already trimmed and merged.
For the VLC/ARS/DLV/PLV/RIG/OFN samples, and some of the blanks and deep sequenced samples that came later, I ran the same as below, but with FastQC and AdapterRemoval turned on with default settings.
Organism: Human
Age: Ancient
Treated Data: non-UDG [Deep : UDG]
Pairment: Single End (VLC/EXB/LIB - Paired End)
Input is already concatenated (skip merging): Y (VLC/EXB/LIB - N)
Concatenate Lanewise together: N (VLC -Y, EXB/LIB - N)
Reference: HG19
Name of mitocondrial chromosome: chrMT
FastQC: Off (VLC/EXB/LIB/ARS - On)
AdapterRemoval: Off (VLC/EXB/LIB - On)
Mapping: BWA
Seedlength: 32
Max # diff: 0.01
Qualityfilter: 0
Filter unmapped: Off
Extract Mapped/Unmapped: On
Remove Duplicates: DeDup
Treat all reads as merged: On
Damage Calculation: Damageprofiler
CleanUp: On
Create Report: On
These settings can be seen in table format in 06-additional_data_files
under Data R05.
The EAGER runs were be submitted with:
EAGER=<PATH_TO>/1.92.55/bin/eager
EAGERCLI=<PATH_TO>/1.92.55/bin/eagercli
for FILE in $(find 03-preprocessing/screening/human_filtering/output/* -name '*.xml'); do
unset DISPLAY && $EAGERCLI $(readlink -f $FILE)
done
For the screening data to clean up the EAGER results directories so that they are in the same format as the script version others we ran the following couple of commands.
All production dataset files were run with EAGER, so clean up was not required and the default ReportTable output was used for statistics reporting
To run clean-on on single libraries:
cd 03-preprocessing/screening/human_filtering/output
## Clean up
SAMPLE=LIB007.A0124
SAMTOOLS=<PATH_TO>/samtools_1.3/bin/samtools
for DIR in $(readlink -f "$SAMPLE"); do
cd "$DIR"
mkdir fastqc
mv 0-FastQC/*zip fastqc
mv 1-AdapClip/*settings .
mv 4-Samtools/*mapped.bam.stats .
mv 4-Samtools/*extractunmapped.bam .
mv 5-DeDup/*.mapped.sorted.cleaned.hist .
mv 5-DeDup/*.log .
mv 6-QualiMap/*/ qualimap
mv 7-DnaDamage/*/ damageprofiler
done
cd ..
for DIR in $(readlink -f "$SAMPLE"); do
cd "$DIR"
"$SAMTOOLS" idxstats $(readlink -f 4-Samtools/*.mapped.sorted.bam) >> $(readlink -f 4-Samtools/*.mapped.sorted.bam).idxstats
mv 4-Samtools/*idxstats .
rm -r 0-FastQC
rm -r 1-AdapClip
rm -r 3-Mapper
rm -r 4-Samtools
rm -r 5-DeDup
rm -r 6-QualiMap
rm -r 7-DnaDamage
rm DONE.CleanUpRedundantData
rm EAGER.log
done
And for multiple samples:
SAMPLES=($(find -name '2019*.xml' -type f -exec readlink -f {} \;))
SAMTOOLS=<PATH_TO>/samtools_1.3/bin/samtools
for DIR in ${SAMPLES[@]}; do
cd $(dirname "$DIR")
mkdir fastqc
mv 0-FastQC/*zip fastqc
mv 1-AdapClip/*settings .
mv 4-Samtools/*mapped.bam.stats .
mv 4-Samtools/*extractunmapped.bam .
mv 5-DeDup/*.mapped.sorted.cleaned.hist .
mv 5-DeDup/*.log .
mv 6-QualiMap/*/ qualimap
mv 7-DnaDamage/*/ damageprofiler
done
cd ..
for DIR in ${SAMPLES[@]}; do
cd $(dirname "$DIR")
"$SAMTOOLS" idxstats $(readlink -f 4-Samtools/*.mapped.sorted.bam) >> $(readlink -f 4-Samtools/*.mapped.sorted.bam).idxstats
mv 4-Samtools/*idxstats .
rm -r 0-FastQC
rm -r 1-AdapClip
rm -r 3-Mapper
rm -r 4-Samtools
rm -r 5-DeDup
rm -r 6-QualiMap
rm -r 7-DnaDamage
rm DONE.CleanUpRedundantData
rm EAGER.log
done
⚠️ The per-sample EAGER output files are not provided here due to their large size.
As EAGER itself does not have ability to convert unmapped read BAMs to FASTQ, we manually ran this for EAGER-preprocessed files with:
FILES=($(find -L 03-preprocessing/{screening,deep}/human_filtering/output/{FUM,GOY,PES,GDN}*.2/ -name '*.extractunmapped.bam' -type f))
for i in 1:${#FILES[@]}; do
samtools fastq ${FILES[$i]} | gzip > ${FILES[$i]} .fq.gz;
done
⚠️ The unmapped-read FASTQ files are not provided here due to their large size.
We then extracted the statistics of this pre-processing with the script
02-scripts.backup/005-statistics_human_filtering.sh. Once checked, we moved
the resulting human_filtering_statistics.csv output file to our 00-documents
folder and renamed as 03-human_filtering_statistics.csv.
SCRIPTS=02-scripts.backup
cd 03-preprocessing/screening/human_filtering
"$SCRIPTS"/005-statistics_human_filtering.sh output/
## Copy to documentation folder
mv human_filtering_statistics_"$(date +"%Y%m%d")".csv ..
cp ../human_filtering_statistics_"$(date +"%Y%m%d")".csv ../../../00-documentation.backup/03-human_filtering_statistics_"$(date +"%Y%m%d")".csvNote: if you get a "Runtime error (func=(main), adr=6): Divide by zero" error, don't worry, this is related to the cluster factor calculation for when there are no human reads after de-duplication. I just manually filled these in with NA.
The summarised results from this preprocessing can be seen in
06-additional_data_files under Data R06
For the deep sequenced data, the EAGER table was used for report statistics (see
below forfurther information), and can be seen in 06-additional_data_files
under Data R08.
Next, we merged together libraries that were sequenced multiple times, or individuals with multiple calculus samples.
A list of libraries that have been merged together can be seen for the screening
data at 06-additional_data_files under Data R07 or
00-documentation/04-library_merging_information.csv. For the production
dataset the same is either under Data R09 or
at 00-documentation/17-samples_deep_library_merging_information_20190708.csv.
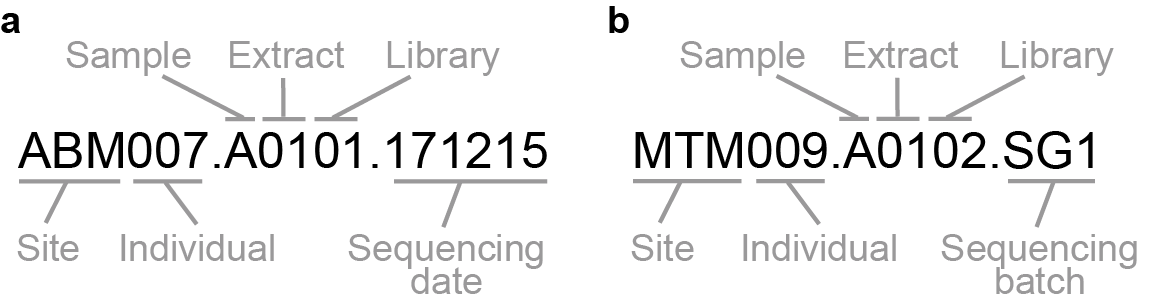
The structure of the MPI-SHH sample naming system can be seen here:
Library merging in this case can therefore be worked out by merging together any library that shares the first six character section of each library. For example:
CDC005.A0101
CDC005.A0101.170817
or
ECO002.B0101
ECO002.C0101
The two examples represent distinct individuals (but different libraries of a
single sample in the case of different .A characters or multiple sequencing
of the same library in the case of the date string .170817). In this study
we did not consider possible differences in sampling tooth source of the
calculus, and therefore pooled accordingly either at sampling or
in silico here.
First, into our final output preprocessing directory (03-preprocessing/*/library_merging/output),
we quickly imported all files for all individuals as symlinks
cd 03-preprocessing/screening/library_merging
## Make directory
for DIR in ../../screening/human_filtering/output/*/; do
mkdir "$(echo $DIR | rev | cut -d/ -f2 | rev)/"
done
## Make symlink of FastQ into new directory above.
for DIR in ../../screening/human_filtering/output/*/; do
ln -s $(readlink -f "$DIR")/*fq.gz "$(echo $DIR | rev | cut -d/ -f2 | rev)/";
doneThen we removed the imported directories of 'extra' samples/libraries that needed to be merged.
rm \
ABM007.A0101/*.fq.gz \
ABM008.A0101/*.fq.gz \
BIT001.A0101/*.fq.gz \
CDC005.A0101/*.fq.gz \
CDC011.A0101/*.fq.gz \
DJA006.A0101/*.fq.gz \
DJA007.A0101/*.fq.gz \
EBO008.A0101/*.fq.gz \
EBO009.A0101/*.fq.gz \
ECO002.B0101/*.fq.gz \
ECO004.B0101/*.fq.gz \
FDM001.A0101/*.fq.gz \
GDN001.A0101/*.fq.gz \
IBA001.A0101/*.fq.gz \
IBA002.A0101/*.fq.gz \
LOB001.A0101/*.fq.gz \
MOA001.A0101/*.fq.gz \
MTK001.A0101/*.fq.gz \
MTM003.A0101/*.fq.gz \
MTM010.A0101/*.fq.gz \
MTM011.A0101/*.fq.gz \
MTM012.A0101/*.fq.gz \
MTM013.A0101/*.fq.gz \
MTS001.A0101/*.fq.gz \
MTS002.A0101/*.fq.gz \
MTS003.A0101/*.fq.gz \
TAF017.A0101/*.fq.gz \
TAF018.A0101/*.fq.gz \
WAL001.A0101/*.fq.gzThese extras were then merged into a single FASTQ file for each individual using
cat, and placed in an independent file in the output directories with the
following commands
INDIR=03-preprocessing/screening/human_filtering/output
OUTDIR=03-preprocessing/screening/library_merging
cat "$INDIR"/ABM007*/*.fq.gz >> "$OUTDIR"/ABM007.A0101/ABM007_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/ABM008*/*.fq.gz >> "$OUTDIR"/ABM008.A0101/ABM008_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/BIT001*/*.fq.gz >> "$OUTDIR"/BIT001.A0101/BIT001_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/CDC005*/*.fq.gz >> "$OUTDIR"/CDC005.A0101/CDC005_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/CDC011*/*.fq.gz >> "$OUTDIR"/CDC011.A0101/CDC011_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/DJA006*/*.fq.gz >> "$OUTDIR"/DJA006.A0101/DJA006_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/DJA007*/*.fq.gz >> "$OUTDIR"/DJA007.A0101/DJA007_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/EBO008*/*.fq.gz >> "$OUTDIR"/EBO008.A0101/EBO008_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/EBO009*/*.fq.gz >> "$OUTDIR"/EBO009.A0101/EBO009_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/ECO002*/*.fq.gz >> "$OUTDIR"/ECO002.B0101/ECO002_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/ECO004*/*.fq.gz >> "$OUTDIR"/ECO004.B0101/ECO004_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/FDM001*/*.fq.gz >> "$OUTDIR"/FDM001.A0101/FDM001_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/GDN001*/*.fq.gz >> "$OUTDIR"/GDN001.A0101/GDN001_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/IBA001*/*.fq.gz >> "$OUTDIR"/IBA001.A0101/IBA001_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/IBA002*/*.fq.gz >> "$OUTDIR"/IBA002.A0101/IBA002_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/LOB001*/*.fq.gz >> "$OUTDIR"/LOB001.A0101/LOB001_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/MOA001*/*.fq.gz >> "$OUTDIR"/MOA001.A0101/MOA001_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/MTK001*/*.fq.gz >> "$OUTDIR"/MTK001.A0101/MTK001_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/MTM003*/*.fq.gz >> "$OUTDIR"/MTM003.A0101/MTM003_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/MTM010*/*.fq.gz >> "$OUTDIR"/MTM010.A0101/MTM010_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/MTM011*/*.fq.gz >> "$OUTDIR"/MTM011.A0101/MTM011_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/MTM012*/*.fq.gz >> "$OUTDIR"/MTM012.A0101/MTM012_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/MTM013*/*.fq.gz >> "$OUTDIR"/MTM013.A0101/MTM013_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/MTS001*/*.fq.gz >> "$OUTDIR"/MTS001.A0101/MTS001_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/MTS002*/*.fq.gz >> "$OUTDIR"/MTS002.A0101/MTS002_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/MTS003*/*.fq.gz >> "$OUTDIR"/MTS003.A0101/MTS003_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/TAF017*/*.fq.gz >> "$OUTDIR"/TAF017.A0101/TAF017_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/TAF018*/*.fq.gz >> "$OUTDIR"/TAF018.A0101/TAF018_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
cat "$INDIR"/WAL001*/*.fq.gz >> "$OUTDIR"/WAL001.A0101/WAL001_S0_L000_R1_000.fastq.merged.prefixed.hg19unmapped.fq.gz
⚠️ All merged FASTQ are files not provided here due to their large size.
For screening data, the final number of reads going downstream analysis is also
recorded in the file 04-samples_library_merging_information.csv. For libraries
sequenced twice (i.e. has .SG.2 or a date, I manually added the two values
together.
For deep sequencing data, The same concatenating of multiple samples and/or
lanes was done for the deep sequencing samples. However the statistics were
summarised across multiple libraries using the R notebook
02-scripts.backup/099-eager_table_individual_summarised.Rmd.
Visualisation of summary statistics can also be seen below.
The human DNA GC content could be a bit off in some of the new libraries generated in this study, as we mostly sequenced with Illumina NextSeqs. These have a 2 colour chemistry that considers no light emission as a 'G'. Thus, empty or finished clusters can be read as long poly G reads - which can still map to the human genome in regions with long repetitive regions. Modern contamination with long human DNA reads on a failed flow cell cluster may also containing poly-G stretches if the florescence failed before the entire read is complete and the adapter had not been sequenced.
The polyG issue would not likely affect our ancient microbial data because these should be so short that each read would have both ends of the read entirely sequenced (the whole read with both adapters or most of both adapters would fit in 75 cycles). Thus, adapter removal would remove anything that comes after the adapters which would be the poly Gs. Poly G tails would only affect single index reads, where the read itself is too short and doesn't have an adapter to indicate the read has ended.
To get improved human DNA content calculations, we performed the following steps,
the output of which was stored in 04-analysis/screening/eager under the
polyGremoval* directories.
-
AdapterRemoval with no quality trimming or merging, just adapter removal
sbatch 02-scripts.backup/099-polyGcomplexity_filter_preFastPprep.sh
-
Clean up the output in
04-analysis/screening/eager/polyGremoval_input/output/withfind . ! -name '*fastq.gz' -type f -delete
-
Run FastP on output of AdapterRemoval with
sbatch 02-scripts.backup/099-polyGcomplexity_filter.sh
-
Finally run the output of FastP again with EAGER. Note that we still need to run AR to merge reads, but it's not important for re-clipping as there shouldn't be any adapters, so will put minimum adapter overlap to 11 (EAGER doesn't allow merging only)
Organism: Human Age: Ancient Treated Data: non-UDG Pairment: Single End (R1) or Paired End (R1/R2) Input is already concatenated (skip merging): N Concatenate Lanewise together: N (as lane concatenation already done) Reference: HG19 Name of mitochondrial chromosome: chrMT AdapterRemoval: N minimum adapter overlap: 11 Mapping: BWA Seedlength: 32 Max # diff: 0.01 Qualityfilter: 0 Filter unmapped: On Extract Mapped/Unmapped: Off Remove Duplicates: DeDup Treat all reads as merged: Y Damage Calculation: Y CleanUp: On Create Report: On
To run each EAGER run in parallel, we again used
02-scripts.backup/02-scripts.backup/021-eager_microbiota_slurm_array.sh after
updating the path in the find command in the script to point to the
corresponding EAGER XML directory.
The resulting human mapping data after poly-G removal can be seen in
00-documentation.backup/99-PolyGRemoved_HumanMapping_EAGERReport_output.csv
Visualisation of summary poly-G trimming statistics can also be seen below.
⚠️ The EAGER run output files are not provided here due to their large size.
Sequencing quality control results for both screening and production datasets
were generated in the script seen in
02-scripts.backup/099-SequencingQCMetrics_v2.Rmd.
The following figures also describe a subset of these preprocessing summary statistics.
Figure R1 | Sequencing metric distributions of the screening dataset of ancient and modern calculus samples newly sequenced during this study. Each point represents a single individual (i.e. all samples, libraries and re-sequencing runs combined). a Raw sequencing read counts (prior to adapter removal and merging). b Pre-processed read counts after adapter removal and read merging. c Proportion of human DNA. d Count of non-human reads used for downstream analysis (pre-processed reads with human sequences removed).
Figure R2 | Sequencing read count distributions of the production dataset of ancient calculus samples newly sequenced during this study. Each point represents a single indiviudal (i.e. all samples, libraries and re-sequencing runs combined). a Raw sequencing reads (prior adapter removal and merging), b Number of reads used for downstream analysis (processed reads with human sequences removed).
The general metadata file for all main individual-level pre-processing
statistics can be seen in 06-additional_data_files under Data R04. This file
is typically used as input for all downstream analyses, when required.
For the first step of analysing the microbiome content of dental calculus, controls and comparative samples is to perform taxonomic binning and classification. This allows us to rapidly identify what species are present in each library.
For taxonomic binning of reads of the screening dataset, we used MALT with
a wrapper script for efficient submission. The settings set in
02-scripts/007-malt-genbank-nt_2017_2step_85pc_supp_0.01 are as follows:
- a read requires a minimum of 85% sequence identity (
-id), - it will only retain a second hit if the alignment is within 1% of the
bit-score of the top scoring hit (
-top) - it will only keep a leaf-node on the tree if it has more than 0.01 of the hits
over all the hits in the sample (
-supp). - it will only retain 100 possible hits per node.
Note that you may have to change the
INSTALL4J_JAVA_HOMEpath within the script.
We then ran our taxonomic binning job with the following command, providing the input directory and a wild-cards to pick up all merged files to individual level, and an output directory.
02-scripts.backup/007-malt-genbank-nt_2017_2step_85pc_supp_0.01 \
03-preprocessing/screening/library_merging/*/*.fq.gz \
04-analysis/screening/malt/ntThis script aligns to the NCBI Nucleotide database (nt). For the CustomRefSeq database, see below
⚠️ The RMA6 files are not provided here due to their large size.
To extract the summary statistics for all the MALT runs, we ran the following command on the MALT logs produced by the script described above.
grep -e "Loading MEGAN File:" \
-e "Total reads:" \
-e "With hits:" \
-e "Alignments:" \
-e "Assig. Taxonomy" \
-e "Min-supp. changes" \
-e "Numb. Tax. classes:" \
-e "Class. Taxonomy:" \
-e "Num. of queries:" \
-e "Aligned queries:" \
-e "Num. alignments:" \
-e "MinSupport set to:" \
04-analysis/screening/malt/nt/*log | cut -d":" -f 2-99 > 00-documentation.backup/99-maltAlignedReadsSummary_raw_nt_$(date "+%Y%m%d").txtNote: if you have failed runs or single samples, and repeat the command, check to remove those 'failed' entries after running the grep command
To clean up the output of the grep command, and perform additional
calculation steps, we ran the following R
script: 02-scripts.backup/099-MALT_Summary_statistics.R, the output of which
is recorded in
00-documentation.backup/05-MALT_taxonomic_binning_summary_statistics_nt.tsv
for nt and for RefSeq in
00-documentation.backup/05-MALT_taxonomic_binning_summary_statistics_refseq_bacharchhomo_gcs_20181122.tsv
The MinSupport value column(s) was then manually added to the individuals
column of our the final main screening metadata file
00-documentation/02-calculus_microbiome-deep_evolution-individualscontrolssources_metadata_20190523.tsv.
Summary statistics on the number of reads assigned per individual to the MALT and RefSeq databases can be seen below
Once MALT had completed, we needed to generate an OTU table of all the alignments.
For this we used the GUI tool MEGAN6 that MALT is based on our local machine. To generate the OTU table, we opened the MALT RMA6 files in MEGAN with absolute counts, and ignored all unassigned reads.
To un-collapse the tree we pressed the following in the tool-bar: Tree > Rank > <Taxonomic level>, the then select species nodes with: Select > Rank > <Taxonomic level>.
Then, File > Export > Text (CSV) format, selected taxonName_to-count, summarised and tab as a separator.
For a 'microbial-only' OTU table (excluding prokaryotes and synthetic DNA sequences), we un-collapsed the tree by firstly selecting 'collapse non-prokaryotes' under the 'Tree' menu, and then Select > Rank > <taxonomic level> to select only Bacteria and Archaea. We also exported the a tree based on the same data with the option: File > Export > Tree, and saved in Newick format. This was also done for each prokaryote taxonomic level.
These were be saved in 04-analysis/screening/megan.backup, but additionally
the corresponding .megan, .nwk and OTU tables at various taxonomic
levels (as exported by MEGAN) for both Nt and RefSeq
databases can be seen in 06-additional_data_files under Data R11.
For faster accessibility to OTU tables at different stages of downstream
analysis, we made raw MALT OTU tables with and without badly-preserved
individuals (see below) and at
different min. support values (see below)
were generated by the Notebook
02-scripts.backup/016-MALT_otutable_generation.Rmd. These tables are stored
as .tsv files in the 04-analysis/screening/megan.backup
directory as well 06-additional_data_files under Data R11.
We were also interested in whether there were any overall patterns between the samples in terms of broad taxonomic content.
Visualisation and statistical testing of whether the ratio of prokaryotic to
eukyarotic reads differs between well-preserved and badly-preserved samples can
be seen in 02-scripts.backup/099-cumulativedecay_vs_sourcetracker.Rmd.
Visualisation of this analysis can be seen
below
To get an overview of the 'mapability' of the (merged) samples used in this, we
generated summary statistics of numbers of reads taxonomically assigned, as well
as made comparison between the two MALT databases. This was performed with the
R notebook 02-scripts.backup/099-MALTAssignmentResults.Rmd.
Summary figures are below. Please see the main publication for further discussion.
Figure R3 | Comparison of the mean number of taxonomically assigned non-human reads across all sources, laboratory controls and comparative sample groups. Taxonomic assignment is from aligning to the NCBI nt (2017) and a custom NCBI refseq (2018) databases using MALT. Colours correspond to calculus host genus. Blue: Alouatta; Purple: Gorilla; Green: Pan; Orange: Homo; Grey: non-calculus.
Figure R4 | Gardner-Altman plot showing differences in the mean percent of taxonomically assigned reads between the NCBI nt (2017) and a custom NCBI RefSeq (2018) databases using MALT. Right hand plot: dot represents mean difference between the two groups with resampling distribution (5000 bootstraps). Left hand plot: slope graph showing relationship between means percent taxonomically assigned reads between two databases per group. Vertical dark line on dot represents 95% confidence interval around the mean. Y axis represents mean percentage of taxonomically assigned reads within each group. a Comparison of overall mean between all calculus samples, laboratory controls and comparative sources in this study. b Comparison of just dental calculus samples.
Figure R5 | Comparison of mean percent of taxonomically assigned reads to different groups of humans, when aligning between the NCBI nt (2017) and a custom NCBI RefSeq (2018) database using MALT. Ancient sample groups are 'pre-agricultural' and 'pre-antibiotic' humans and are taken from skeletal remains, whereas Modern Day Human calculus and HMP plaque samples come from living individuals. Colours correspond to sample type. Orange: Homo calculus; Grey: non-calculus.
We also visualised the prokaryotic over eukaryotic content. See the main publication and below for further discussion of these observations.
Figure R6 | Comparison of ratios of alignments to Bacterial/Archaeal/Viral reference sequences vs. Eukaryotic reference sequences between all calculus, laboratory controls and comparative sources. Ratios are based on the number of reads aligned the NCBI nt (2017) database using MALT. Y-axis is log10 scaled. Colours correspond to calculus host genus. Colours correspond to calculus host genus. Blue: Alouatta; Purple: Gorilla; Green: Pan; Orange: Homo; Grey: non-calculus.
Figure R7 | Comparison of ratios of alignments to Bacterial/Archaeal/Viral reference sequences vs. Eukaryotic reference sequences between different groups of humans. Ratios are based on the number of reads aligned the NCBI nt (2017) database using MALT. Y-axis is log10 scaled. Ancient sample groups are 'pre-agricultural' and 'pre-antibiotic' humans and are taken from skeletal remains, whereas Modern Day Human calculus and HMP plaque samples come from living individuals. Colours correspond to calculus host genus. Colours correspond to calculus host genus. Orange: Homo; Grey: non-calculus.
A crucial part of any ancient DNA study is to control for preservation of the DNA content of any analysed samples. Due to taphonomic processes, the original endogenous DNA can be come very degraded and also entirely lost. This can lead to samples containing only contaminant DNA and thus cause major skews and complications in downstream analysis. Identifying well-preserved samples containing a sufficient fraction of the original microbiome is critical, but equally so is assessing that their DNA is not derived from modern contamination.
The MALT OTU table(s) alone does not give us much information about the genetic preservation of the original oral signature within each individual.
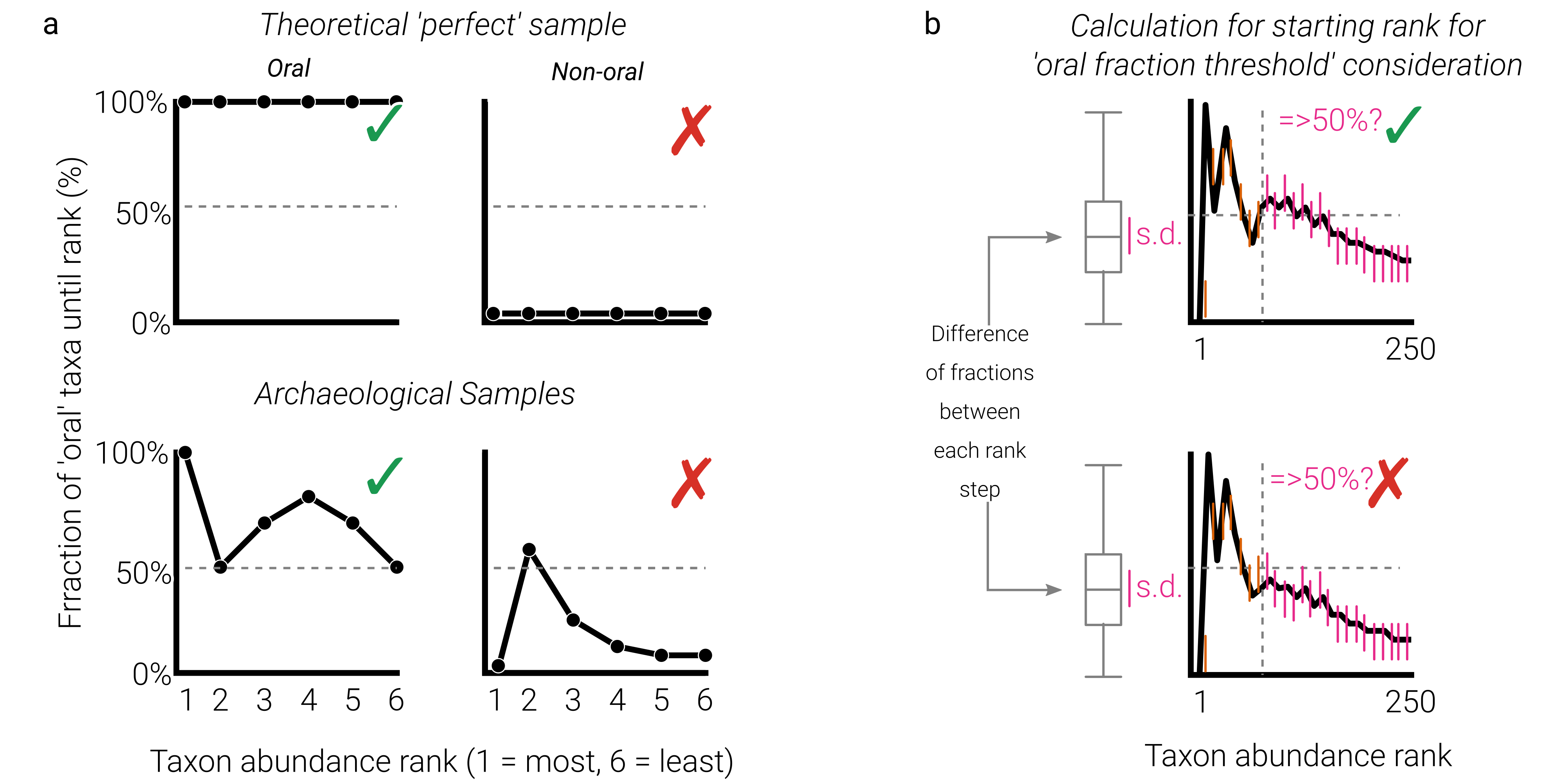
To get a rapid idea of the level of identifiable oral taxa in each individual, I came up with a simple visualisation to show how abundant the (hpoefully endogenous) oral signal in the samples are. It is based on the decay of the fraction of oral taxa identified when looking from most to least abundant taxa within a sample. The concept is further described in the main publication supplement, but a schematic on how to interpret them can be seen here:
Figure R8 | Schematic diagram of a cumulative percent decay method of preservation assessment. a example curves of a theoretical sample consisting of purely oral taxa (top left), and a theoretical sample containing no oral taxa (top right). For the archaeological samples, a well-preserved sample (bottom left) will consist mostly of oral taxa but may include uncharacterised or contaminant taxa leading to a non-linear relationship, but remaining above an oral taxon fraction percent of 50% (as identified in modern plaque samples). An archaeological sample (bottom right) with no endogenous oral content will have few oral taxa, and may have occasional modern contaminants resulting in a non-linear relationship. b a representation of the method for calculating the rank from which to begin assessing whether a sample decay curve goes above the 'well-preserved' fraction threshold, accounting for high variation in mixed preservation samples with both oral and non-oral/uncharacterised taxa at higher ranks. Given the large differences between the initial ranks due to small denominators, a 'burn-in' like procedure is applied. The rank at which the difference change between each subsequent rank does not exceed the standard deviation of all rank differences, is set as the rank from which, it is assessed whether the sample curve exceeds the preservation threshold (here 50% for the NCB nt OTU table). A curve that does not exceed this threshold at any point from this rank onwards, is considered not to have sufficient preservation for downstream analysis.
This visualisation requires two objects: an OTU table from MEGAN at species level (generated above) and a database of taxa with their 'sources'.
To generate the database of oral taxa, I used the steps outlined in the
notebook
02-scripts.backup/013-Organism_Isolation_Source_Database_Generation.Rmd.
Note that database also requires manual curation over time as more taxa are identified and the isolation sources reported.
This database used in this study can be
seen under 06-additional_data_files under Data R16, or stored under
00-documentation as 07-master_oralgenome_isolationsource_database.tsv
To actually generate the visualisations, the R notebook
02-scripts.backup/014-cumulative_proportion_decay_curves.Rmd describes how to
calculate the percent decay curves, including burn-in calculations, and how to
display the curves.
The resulting list(s) of individuals passing or failing to pass this threshold
can be seen in 06-additional_data_files under Data R17 or
04-analysis/screening/cumulative_decay.backup, and summaries of how many
samples passed the calculated threshold in the tables below.
Figure R9 | Cumulative percent decay plots of fraction of oral taxa across taxa ordered by abundance rank. Taxonomic assignment against the: a NCBI nt database, and b a custom NCBI RefSeq database showing a large number of calculus samples displayed greater levels of preservation (blue), and although a smaller number do not pass the estimated preservation threshold (red). Plots are limited to 250 rank positions (x-axis) for visualization purposes. Thresholds are selected based on observations that all sources and controls do not increase about 50% (nt) and 65% (RefSeq) of fraction of oral taxon. Point at which the per-sample threshold is considered is based on when the fluctuation of the fraction of oral taxa (i.e. fraction difference between a taxon and next abundant taxon) does not exceed the standard deviation of all differences of the rank.
Table R1 | Summary of sample counts passing preservation thresholds as implemented in the cumulative percent decay method. Preservation was assessed using the cumulative percent decay method with the fluctuation burn-in threshold based on the MALT OTU tables.
| Database | Host Group | Passed | Failed | Total Samples (n) | Passed (%) |
|---|---|---|---|---|---|
| nt | Howler | 5 | 0 | 5 | 100 |
| nt | Gorilla | 14 | 15 | 29 | 48.28 |
| nt | Chimp | 16 | 5 | 21 | 76.19 |
| nt | Neanderthal | 7 | 10 | 17 | 41.18 |
| nt | PreagriculturalHuman | 16 | 4 | 20 | 80 |
| nt | PreantibioticHuman | 13 | 1 | 14 | 92.86 |
| nt | ModernDayHuman | 18 | 0 | 18 | 100 |
| refseq | Howler | 5 | 0 | 5 | 100 |
| refseq | Gorilla | 12 | 17 | 29 | 41.38 |
| refseq | Chimp | 11 | 10 | 21 | 52.38 |
| refseq | Neanderthal | 7 | 10 | 17 | 41.18 |
| refseq | PreagriculturalHuman | 11 | 9 | 20 | 55 |
| refseq | PreantibioticHuman | 13 | 1 | 14 | 92.86 |
| refseq | ModernDayHuman | 18 | 0 | 18 | 100 |
Table R2 | Comparison of number of individuals with supported good- or low- preservation assignment between the nt and custom RefSeq database. Preservation was assessed using the cumulative percent decay method with the fluctuation burn-in threshold based on the MALT OTU tables.
| Population | Total Individuals in Population | Individuals with Matching Preservation Assignment | Individuals not Matching Preservation Assignment |
|---|---|---|---|
| Chimp_2 | 7 | 6 | 1 |
| Chimp_3 | 6 | 4 | 2 |
| Chimp_4 | 1 | 1 | 0 |
| Gorilla_1 | 15 | 13 | 2 |
| Gorilla_2 | 8 | 7 | 1 |
| Gorilla_3 | 6 | 5 | 1 |
| Howler_Monkey | 5 | 5 | 0 |
| ModernDayHuman_1 | 10 | 10 | 0 |
| ModernDayHuman_2 | 8 | 8 | 0 |
| Neanderthal | 17 | 15 | 2 |
| PreagriculturalHuman_1 | 10 | 8 | 2 |
| PreagriculturalHuman_2 | 10 | 7 | 3 |
| PreantibioticHuman_1 | 10 | 10 | 0 |
| PreantibioticHuman_2 | 4 | 4 | 0 |
We next wanted to compare our screening method to a less-suitable but more established approach, Sourcetracker analysis. However this typically uses an OTU table of 16S rRNA reads to estimate the proportion of OTUs derived from the sources that are also in your sample.
As we have shotgun data, we instead extracted reads with sequence similarity to translated 16S rRNA sequences and ran on that.
The script 02-scripts.backup/009-preprocessing-16s_mapping works very much
the same way as with the human DNA preprocessing, but
mapping to the SILVA database and converting the unmapped-only reads (i.e.
non-human reads from the preprocessing) to FASTA
format, with a particular header format (>sample.name_1) that is
compatible with QIIME.
INDIR=02-scripts.backup/03-preprocessing/screening/library_merging
OUTDIR=02-scripts.backup/03-preprocessing/screening/silva_16s_reads
SCRIPTS=02-scripts.backup/02-scripts.backup
for LIBDIR in "$INDIR"/*/; do
LIBNAME=$(echo "$LIBDIR" | rev | cut -d/ -f2 | rev)
if [ -d "$OUTDIR"/"$LIBNAME" ]; then
printf "\n $LIBNAME already Processed \n\n"
else
mkdir "$OUTDIR"/"$LIBNAME"
$SCRIPTS/009-preprocessing_16s_mapping.sh $OUTDIR/$LIBNAME $LIBDIR $LIBNAME
sleep 1
fi
doneTo get the number of 16S rRNA reads that mapped, we can ran script
02-scripts.backup/06-16s_extraction_statistics.sh, and copied the resulting
file into our documents directory with
SCRIPTS=02-scripts.backup/02-scripts.backup
cd 02-scripts.backup/03-preprocessing/screening
## Generate stats
"$SCRIPTS"/011-16s_extraction_statistics.sh silva_16s_reads/
## Copy into documentation folder
mv silva_16s_reads/16s_extraction_statistics_"$(date +"%Y%m%d")".csv .
cp 16s_extraction_statistics_"$(date +"%Y%m%d")".csv ../../00-documentation.backup/05-16s_extraction_statistics_"$(date +"%Y%m%d")".csvThe mapping results files are not provided here due to their large size.
The summary statistics for the number of 16S reads identified can be seen in
06-additional_data_files under Data R12 or
00-documentation.backup/05-16s_extraction_statistics.csv, as well as in the
figures below.
Figure R10 | Distributions of percentages of 16S rRNA mapping reads extracted out of all non-human reads across all samples in the dataset, when mapping to the SILVA database. Colours correspond to calculus host genus. Blue: Alouatta; Purple: Gorilla; Green: Pan; Orange: Homo; Grey: non-calculus.
Figure R11 | Distributions of percentages of 16S rRNA mapping reads extracted out of all non-human reads libraries across human calculus and plaque samples by mapping to the SILVA database. Ancient sample groups are 'pre-agricultural' and 'pre-antibiotic' humans and are taken from skeletal remains, whereas Modern Day Human calculus and HMP plaque samples come from living individuals. Colours correspond to sample type. Orange: Homo calculus; Grey: non-calculus.
Summary statistic visualisation of mapping can be seen under
02-scripts.backup/099-16sResults.Rmd
To assign taxonomic classifications to the identified 16S reads, we used then used QIIME (v1.9) to cluster the reads by similarity, and assign a taxonomic 'name'. We remained with using OTU clustering rather than the more recent (and more powerful/statistically valid) ASV (amplicon sequence variant) methods. This is because we are not dealing directly with amplicon data, and we also have damage which would affect reliability of assignment. Finally, the more recent version of QIIME, QIIME2, has been made much less flexible for non-amplicon data (at the time of writing) and I couldn't work out how to adapt the data. For our rough preservational screening purposes using QIIME 1.9 was deemed sufficient.
Firstly, we made a combined FASTA file containing all the 16S reads from all the samples.
INDIR=02-scripts.backup/03-preprocessing/screening/silva_16s_reads
OUTDIR=04-analysis/screening/qiime/input
rm "$OUTDIR"/silva_16s_reads_concatenated.fna.gz
touch "$OUTDIR"/silva_16s_reads_concatenated.fna.gz
for SAMPLE in "$INDIR"/*; do
find "$SAMPLE" -maxdepth 1 -name '*_renamed.fa.gz' -exec cat {} >> \
"$OUTDIR"/silva_16s_reads_concatenated.fna.gz \;
doneThe FASTA file is not provided here due to their large size.
For OTU clustering itself, we additionally defined custom parameters that have
been adapted for shotgun data by LMAMR in Oklahoma. These are stored in the
file 02-scripts.backup/010-params_CrefOTUpick.txt, as well as in the table
below.
Table R3 | Non-default parameters used for QIIME closed-reference clustering.
| Parameter | Value |
|---|---|
| pick_otus:max_accepts | 100 |
| pick_otus:max_rejects | 500 |
| pick_otus:word_length | 12 |
| pick_otus:stepwords | 20 |
| pick_otus:enable_rev_strand_match | TRUE |
To actually run the clustering analysis and generate our OTU table we ran the following.
INDIR=04-analysis/screening/qiime/input
OUTDIR=04-analysis/screening/qiime/output
GREENGENESDB=<PATH_TO>/qiime_default_reference/gg_13_8_otus/
SCRIPTS=02-scripts.backup/02-scripts.backup
gunzip "$INDIR/silva_16s_reads_concatenated.fna.gz"
pick_closed_reference_otus.py \
-i $INDIR/silva_16s_reads_concatenated.fna \
-o $OUTDIR/otu_picking \
-a \
-O 16 \
-r $GREENGENESDB/rep_set/97_otus.fasta \
-t $GREENGENESDB/taxonomy/97_otu_taxonomy.txt \
-p $SCRIPTS/010-qiime_1_9_1_params_CrefOTUpick.txt
Once finished we re-gzipped the input fasta file to save disk space with:
INDIR=04-analysis/screening/qiime/input
pigz -p 4 "$INDIR/silva_16s_reads_concatenated.fna"To get some basic statistics about the OTU picking, we used the BIOM package that came with the QIIME environment.
INDIR=04-analysis/screening/qiime/input
OUTDIR=04-analysis/screening/qiime/output
cd "$OUTDIR"
biom summarize-table -i "$OUTDIR"/otu_picking/otu_table.biom >> \
"$OUTDIR"/otu_picking/otu_table_summary.txt
## Check with:
head "$OUTDIR"/otu_picking/otu_table_summary.txt -n 20This summary can also be seen in 06-additional_data_files under Data R13.
Sourcetracker (I realised later) doesn't do rarefaction properly as it allows sampling with replacement of the OTUS. Therefore, we needed to manually remove samples that have less than 1000 OTUs (which is the default rarefaction level in Sourcetracker - see below). Looking at the table summary in Data R13 summary shows that fortunately this will remove very few samples and will remove mostly blanks.
cd 04-analysis/screening/qiime/output/otu_picking
INDIR=04-analysis/screening/qiime/output/otu_picking
filter_samples_from_otu_table.py \
-i "$INDIR"/otu_table.biom \
-o "$INDIR"/otu_table_1000OTUsfiltered.biom \
-n 1000
biom summarize-table -i "$INDIR"/otu_table_1000OTUsfiltered.biom >> \
"$INDIR"/otu_table_1000OTUsfiltered_summary.txt
## Check with:
head "$INDIR"/otu_table_1000OTUsfiltered_summary.txt -n 20We also only wanted to look at genus level assignments, given species specific IDs could be unreliable due to damage and different mixtures of strain in different individuals.
To filter to Genus (L6) level we then did
cd 04-analysis/screening/qiime/output/otu_picking
INDIR=04-analysis/screening/qiime/output/otu_picking
## For Genus
summarize_taxa.py \
-i "$INDIR"/otu_table_1000OTUsfiltered.biom \
-o "$INDIR" \
-a \
-L 6
## Fix crappy taxon ID that breaks loading into R when running Sourcetracker :+1: due to hanging quote
sed s#\'Solanum#Solanum#g otu_table_1000OTUsfiltered_L6.txt > otu_table_1000OTUsfiltered_L6_cleaned.tsvWith this final OTU table - as seen (in both biom or TSV format) in
06-additional_data_files under Data R14 or
04-analysis/screening/qiime/output/otu_picking - we could now run
Sourcetracker.
Figure R12 | Distributions of the number of OTUs identified after closed-reference clustering of 16S rRNA reads across all calculus, laboratory controls and comparative sources in this study. Clustering was performed in QIIME at 97% identity Colours correspond to calculus host genus. Blue: Alouatta; Purple: Gorilla; Green: Pan; Orange: Homo; Grey: non-calculus.
Figure R13 | Distributions of the number of OTUs identified after closed-reference clustering of 16S rRNA read sequences at 97% sequence similarity in QIIME across human calculus and plaque samples. Ancient sample groups are 'pre-agricultural' and 'pre-antibiotic' humans and are taken from skeletal remains, whereas Modern Day Human calculus and plaque samples come from living individuals. Colours correspond to sample type. Orange: Homo calculus; Grey: non-calculus.
Summary statistic visualisation of clustering was generated via the Rmarkdown
notebook 02-scripts.backup/099-16sResults.Rmd.
With the OTU tables, we are now able to compare the environmental sources (plaque, gut, skin, sediment etc.) to each of the calculus samples and estimate the proportion of each sample that resembles the sources. This thus can help indicate the level of (hopefully) endogenous oral-content preservation in the samples.
Sourcetracker requires an OTU table (generated above) and a metadata file
that tells the program what libraries in the OTU are a 'sink' or a 'source'.
This metadata file used in this case is recorded here,
02-scripts.backup/02-microbiome_calculus-deep_evolution-individualscontrolssources_metadata.tsv,
which I then tried to use for all downstream analysis. In particular here we
needed to ensure there was an 'Env' and a 'SourceSink' column.
To then to run Sourcetracker we ran the following command:
## change to new directory for output directories
INDIR=04-analysis/screening/qiime/output/otu_picking
OUTDIR=04-analysis/screening/sourcetracker.backup/
MAPPINGFILE=00-documentation.backup/02-calculus_microbiome-deep_evolution-individualscontrolssources_metadata_20190429.tsv
Rscript \
sourcetracker_for_qiime.r \
-i "$INDIR"/otu_table_1000OTUsfiltered_L6_cleaned.tsv \
-m "$MAPPINGFILE" \
-o "$OUTDIR"/otu_table_L6_1000_"$(date +"%Y%m%d")" \
-r 1000 \
-v
For plotting of these - with comparison to the cumulative percent decay plots,
I use the following R notebook to summarise the results:
02-scripts.backup/099-cumulativedecay_vs_sourcetracker.Rmd. Discussion
of the comparison can be seen in the main publication, however there was
a generally good concordance between the two approaches.
The final proportions (with standard deviation) can be seen in
06-additional_data_files under Data R18.
Figure R14 | Stacked bar plots representing the estimated proportion of sample resembling a given source, as estimated by Sourcetracker across all calculus samples. Visual inspection shows general concordance between the cumulative percent decay method and Sourcetracker estimation is seen. Coloured label text indicate whether that sample passed (grey) or failed (black) the cumulative percent decay threshold (see above) based on alignments to the NCBI nt (2017) database.
Returning back to the MALT tables and cumulative percent decay plots, we had also observed that the older samples and those with weaker indication of oral content appeared to have a greater ratio of prokaryotic to eukartotic alignments.
We also explored whether this ratio could be used as an additional validation of
oral microbiome preservation in ancient samples. Visualisation and statistical
testing of these ratios can be seen in the figure below, and was generated as
described in the R notebook
02-scripts.backup/099-cumulativedecay_vs_sourcetracker.Rmd. For discussion
of the results, please refer to the main publication.
Figure R15 | Comparison of ratios of bacterial/archaeal/viral over eukaryotic alignments, between ancient calculus samples that passed the cumulative decay cut off for preservation. Samples not passing the preservation threshold as estimated with the cumulative percent decay plots, tend to have smaller ratio and therefore greater amounts of eukaryotic DNA reads being assigned. Ratios are based on the number of reads aligned the NCBI nt (2017) database using MALT.
To rapidly screen for damage patterns indicative of ancient DNA on the observed core microbiome (see below), we ran MaltExtract on all output of MALT, with the core microbiome as input list.
We then developed MEx-IPA to rapidly visualise ancient DNA characteristics across all samples and core taxa.
The results files for this analysis can be seen in
04-analysis/screening/maltExtract/output/AnthropoidsHominidaeHoiminini_core_20190509.
⚠️ the text files in this directory are gzipped and must be decompressed before loading into MEx-IPA!
If you do not wish to clone this whole repository (which is very large),
you can use the following suggestions from
stackoverflow. However, in
case that link doesn't work - the most stable method is to use subversion
(svn).
svn checkout https://github.com/jfy133/Hominid_Calculus_Microbiome_Evolution/trunk/04-analysis/screening/maltExtract/output/AnthropoidsHominidaeHoiminini_core_20190509
find -name '*gz' -type f -exec gunzip {} \;Then copy and paste <path>/<to>/AnthropoidsHominidaeHoiminini_core_20190509
into MEx-IPA to find this.
Example reports for the two oldest Neanderthals (PES001 and GDN001), can be seen below in Figure R16, where for multiple known oral species, both cases display indicative characteristics of true endogenous DNA.
Figure R16 | Example of MEx-IPA reports from the MaltExtract tool of the HOPS pipeline for two Neanderthal individuals, across four known oral microbiome taxa. Individual-Taxon combination shows: C to T misincorporation lesions indicative of DNA deamination; read length distribution with a typical peak <50 bp indicative of fragmented DNA; edit distance and percent identity to the given reference which both show close similarity to the oral reference genome in most cases (1-2 edit distance peak; and 95% identity peak identity). Note that Fretibacterium fastidiosum shows a higher edit distance and low percent identity score, suggesting the reads are likely derived from a relative of that taxon that does not have a genome represented in the database used (NCBI nt 2017).
To generate additional confirmation of damage patterns in oral taxa, the screening data was also mapped to a subset of observed core microbiome reference genomes (see below), using EAGER.
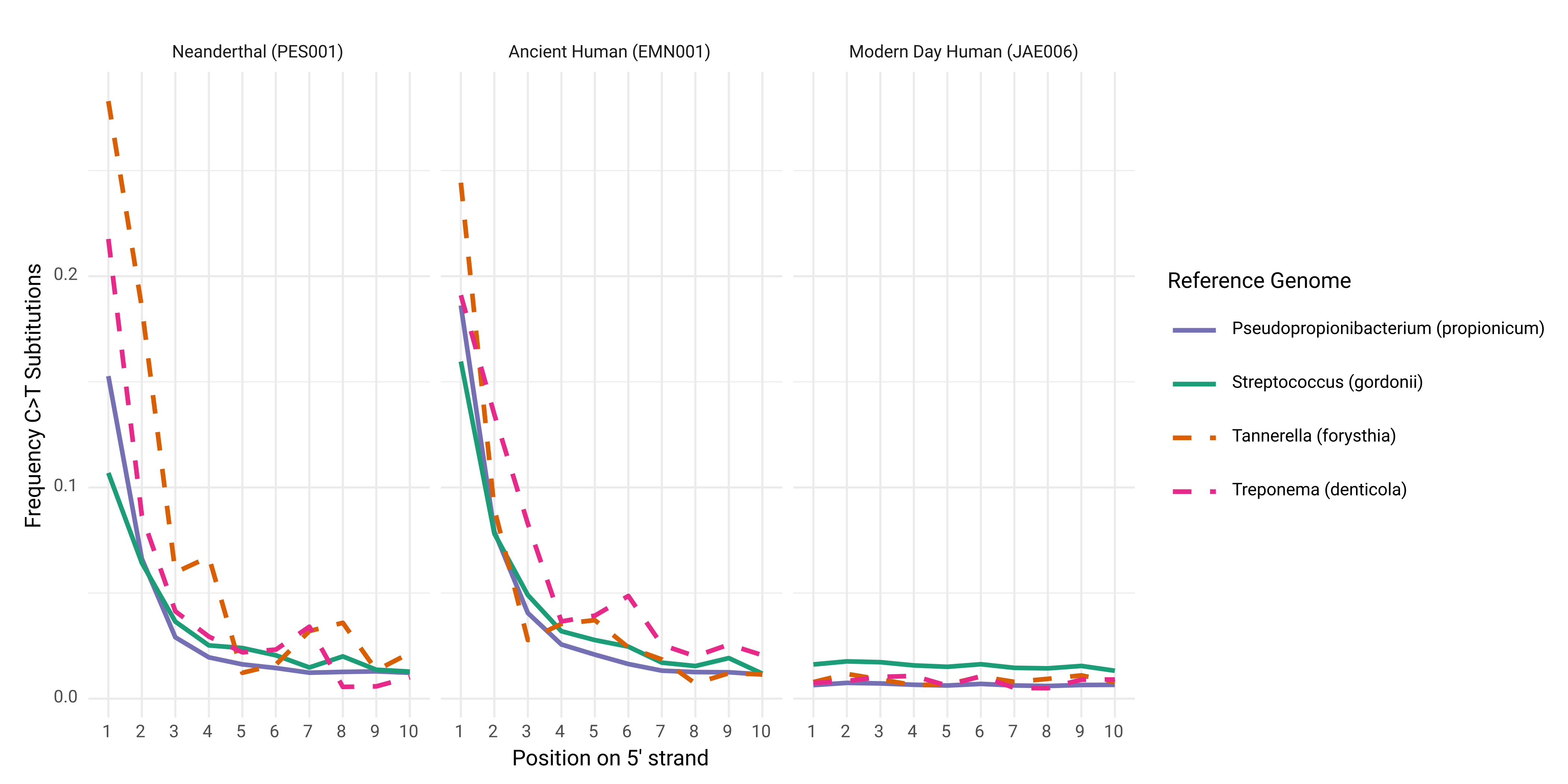
DamageProfiler results were collated and visualised with the R script
02-scripts.backup/099-Coretaxa_SubSet_DamageProfiler_Summary.R. An example of the range of damage signals in ancient Human remains can be seen below in Figure R17. Again showing the presence of multiple well-preserved endogenous DNA of
oral taxa.
Figure R17 | Frequency of C to T miscorporations along 5' ends of DNA reads compared to references of four representative human oral-specific species as calculated by DamageProfiler. Neanderthal and Upper Palaeolithic individuals show damage patterns indicative of authentic aDNA, whereas a modern day individual does not.
The collated results for the whole screening dataset are stored in the file
00-documentation.backup/14-damageprofiler_screening_3p_5p_summaries_20191113.csv.
In addition to identifying well preserved samples, we can also remove possibly
contaminating OTUs from our sample's OTU tables that are derived from the
laboratory environment, we can use the R package decontam. The idea here
is to use this to reduce the number of noisy taxa in the downstream
compositional analysis, e.g. false positive clustering due to laboratory batch
effects.
The method in the decontam package uses library quantification information
to trace inverse correlations in OTU abundance compared to DNA abundance - where
laboratory derived taxa appear more abundant in controls versus true samples.
We manually added the library quantification values (qPCR) to our
main metadata file
02-scripts.backup/02-microbiome_calculus-deep_evolution-individualscontrolssources_metadata.tsv.
We then ran decontam following the decontam tutorial vignette on CRAN as
described here 02-scripts.backup/015-decontam_contamination_detection_analysis.Rmd.
The final list of contaminants for all methods and databases can be seen in
06-additional_data_files under Data R19 and 04-analysis/screening/decontam.backup,
with a summary below in Table R4.
This list of OTUs was subsequently removed from OTU tables in all downstream analyses when indicated.
Table R4 | Summary of OTUs detected across each shotgun taxonomic binner/classifier and databases as potential contaminants by the R package decontam. MetaPhlAn2 was run for functional analysis below. While MetaPhlAn2 was run as reference but wasn't utilised, the contaminants were not removed downstream due to unknown effects of removing these for HUMANn2.
| Binning Method | Database | Taxonomic Level | Total OTUs Detected (n) | Contaminants Detected (n) | Contaminants Detected (%) |
|---|---|---|---|---|---|
| megan | nt | genus | 1392 | 651 | 46.77 |
| megan | nt | species | 3401 | 1557 | 45.78 |
| megan | refseq | genus | 1241 | 630 | 50.77 |
| megan | refseq | species | 5195 | 2183 | 42.02 |
| metaphlan2 | metaphlan2 | genus | 675 | 121 | 17.93 |
| metaphlan2 | metaphlan2 | species | 1626 | 310 | 19.07 |
We observed a high number of putative contaminant OTUs when using our strict
decontam parameters. We wanted to see how much this would provisionally impact
our downstream analyses by investigating how many actual reads the OTUs consist
of a sample. The R notebook
02-scripts.backup/099-decontam_OTU_impact_check.Rmd describes how we did this.
We see that despite large numbers of contaminants are detected by decontam as a fraction of overall OTUs, this only makes up a minority fraction of actual alignments in the MALT OTU table in well preserved samples - suggesting that the majority of contaminants are derived from low-abundant taxa. This is shown in figure R18.
Figure R18 | Fraction of MALT Alignments derived from putative contaminant OTUs show only small effect on well-preserved samples Individuals are ordered by percentage of alignments that are derived from OTUs considered putative contaminants by decontam and removed from downstream analysis. Colour indicates whether the individual was considered to be well-preserved or not based on the cumulative percentage decay curves with the within standard variation burn-in method.
After filtering to contain only well-preserved samples and removing possible contaminants, we then could begin comparison of the calculus microbiomes of our different host groups. We wanted to identify taxonomic similarities and differences between each of the groups to help reconstruct the evolutionary (co-)history of the microbiomes and their hosts.
To explore if we have a structure in our data that can describe differences between each group we want to explore, we performed a Principal Coordinate Analysis to reduce the variation between the samples to human-readable dimensions. An important aspect of this analysis was the use of Compositional Data (CoDA) principles - here implemented with PhILR - which allow us to apply 'traditional' statistics to taxonomic group comparisons.
The steps for the generation of PCoA are described in the R notebook
02-scripts.backup/017-PhILR_PCoA_20190527.Rmd. However, the notebook ending
up having lots of options during parameter exploration (see below). Therefore
I also used knitr::purl()to create a script version of the R notebook that
accepts arguments via the command line.
To generate the script, we ran:
knitr::purl("02-scripts.backup/017-PhILR_PCoA_20190527.Rmd", "02-scripts.backup/017-PhILR_PCoA_20190527_script.R", documentation = 2)Then we generated the PCoAs based on different sets of combinations of variables of:
- Whether to use the NCBI nt database or custom NCBI RefSeq OTU tables
- Whether to analyse at genus or species taxonomic level of taxa in the OTU table
- Whether comparative sources are included or not
- Whether controls are included or not
- Whether to include low preservation samples or not
- Which low preservation samples filtering list to use (see the Cumulative Percentage Decay notebook for further above for futher explanation)
- Either minimum support multipler of 7 for genus or 4 for species (the value in the commands are multipliers of 0.01: the default MALT min. support value set above; the parameters were selected based on the core microbiome calculations below)
The commands for these permutates are therefore as follows:
i=7
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R nt genus withSources withControls in withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R nt genus noSources withControls in withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R nt genus withSources noControls in withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R nt genus noSources noControls in withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R nt genus withSources withControls out withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R nt genus noSources withControls out withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R nt genus withSources noControls out withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R nt genus noSources noControls out withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R refseq genus withSources withControls in withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R refseq genus noSources withControls in withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R refseq genus withSources noControls in withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R refseq genus noSources noControls in withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R refseq genus withSources withControls out withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R refseq genus noSources withControls out withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R refseq genus withSources noControls out withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R refseq genus noSources noControls out withinvariation "$i"
done
i=4
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R nt species withSources withControls in withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R nt species noSources withControls in withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R nt species withSources noControls in withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R nt species noSources noControls in withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R nt species withSources withControls out withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R nt species noSources withControls out withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R nt species withSources noControls out withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R nt species noSources noControls out withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R refseq species withSources withControls in withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R refseq species noSources withControls in withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R refseq species withSources noControls in withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R refseq species noSources noControls in withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R refseq species withSources withControls out withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R refseq species noSources withControls out withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R refseq species withSources noControls out withinvariation "$i"
Rscript 02-scripts.backup/017-PhILR_PCoA_20190527_script.R refseq species noSources noControls out withinvariation "$i"
The R script for summarising the results across all runs is named
017-PhILR_PCoA_summaries.R, and the output files for each combination are in
00-documentation under philr_permanova_*_summary.tsv.
Individual plots of each of the PCoAs and additional considerations can be
seen in 04-analysis/screening/philr.backup More specific comparisons of different parameters can be seen in Figures R19-21. For discussion of the
results, refer to the main publication.
Of particular note, given the sparse nature of our data, we compared between
two zero-replacement methods. This was performed with the script
02-scripts.backup/017-PhILR_PCoA_ZeroReplacementComparison_20190527.Rmd, and
we observed little difference (Figure R19).
Figure R19 | Principal coordinate analysis comparing pseudocount and cumulative zero multiplication zero-replacement methods. Reconstruction of Fig. 1 of main article (but with all sources), shows little differences in the relationships between samples between each method. Scatterplot displays euclidean distances based on genus-level PhILR ratios of all well preserved samples and sources (without controls), putative laboratory contaminants removed, and low abundant taxa removed by a minimum support value of 0.07%. Grey symbols represent comparative sources.
Figure R20 | Validation of low-preservation sample removal with Principal Coordinate Analysis. Visual inspection shows low preservation samples typically fall in compositional ranges of laboratory control or comparative source. PhILR transformed OTU table ordinated by Principal Coordinate Analysis with sources and controls at genus level. Low abundant taxa removed if under 0.07% of overall alignments. a NCBI nt database axis 1 and 2 with low-preservation samples. b NCBI nt database axis 1 and 2 without low-preservation samples. c NCBI nt database axis 2 and 3 with low-preservation samples. d NCBI nt database axis 2 and 3 without low-preservation samples. e Custom NCBI RefSeq database axis 1 and 2 with low-preservation samples. f Custom NCBI RefSeq database axis 1 and 2 without low-preservation samples. g Custom NCBI RefSeq database axis 2 and 3 with low-preservation samples. h Custom NCBI RefSeq database axis 2 and 3 without low-preservation samples. In all cases, samples noted as having low preservation generally fall outside the range of well preserved calculus samples and likely consist of large fractions similar to that of environmental and/or laboratory metagenomic content. Grey symbols represent comparative sources.
Figure R21 | Principal Coordinate Analysis of well-preserved calculus microbiomes at prokaryotic genus taxonomic level by host genus. Visual inspection shows distinct centroids of each host genus, albeit with overlap with others. Input is PhILR transformed OTU tables without sources and controls and low preservation samples removed. Low abundant taxa removed if under 0.07% of overall alignments ('min. support'). a NCBI nt database axis 1 and 2, b NCBI nt database axis 2 and 3, c Custom NCBI RefSeq database axis 1 and 2, and d Custom NCBI RefSeq database axis 2 and 3.
To provide statistical support for the observations made from the PCoAs above
of host groups having putatively distinct calculus microbiomes, we performed
PERMANOVA analysis. This was run in the same R notebook as the PCoAs above
(02-scripts.backup/017-PhILR_PCoA_20190527.Rmd).
The PERMANOVA output related analysis can be seen in Tables R5-7, of which further interpretation can be found in the main text.
Table R5 | Result of PERMANOVA comparing host genera at genus and species level with NCBI nt and custom NCBI RefSeq databases. Calculus microbiomes composition of Gorilla, Pan and Homo are distinct at all taxonomic and database combinations. Alouatta has been removed due to small sample size. Results of PERMANOVA as implemented in the adonis() function in the R package vegan and applied to euclidean distances of filtered and PhILR-transformed MALT alignment OTU tables. Putative laboratory contaminants and badly-preserved samples have been removed. Test statistic is 'pseudo-F'.
| Database | Taxonomic Level | Min Support | Test | Term | Degrees of Freedom | Sum of Squares | Mean of Squares | Test Statistic | R Squared | p value |
|---|---|---|---|---|---|---|---|---|---|---|
| nt | genus | 0.07 | adonis | ind_groups | 3 | 6067420 | 2022473 | 3.3775 | 0.10651 | 0.002 |
| nt | genus | 0.07 | adonis | Residuals | 85 | 50899238 | 598815 | 0.89349 | NA | NA |
| nt | genus | 0.07 | adonis | Total | 88 | 56966658 | 1 | NA | NA | NA |
| nt | species | 0.04 | adonis | ind_groups | 3 | 6659045 | 2219682 | 4.4985 | 0.13702 | 0.001 |
| nt | species | 0.04 | adonis | Residuals | 85 | 41940880 | 493422 | 0.86298 | NA | NA |
| nt | species | 0.04 | adonis | Total | 88 | 48599925 | 1 | NA | NA | NA |
| refseq | genus | 0.07 | adonis | ind_groups | 3 | 7553528 | 2517843 | 2.5456 | 0.09471 | 0.005 |
| refseq | genus | 0.07 | adonis | Residuals | 73 | 72204707 | 989106 | 0.90529 | NA | NA |
| refseq | genus | 0.07 | adonis | Total | 76 | 79758235 | 1 | NA | NA | NA |
| refseq | species | 0.04 | adonis | ind_groups | 3 | 6434342 | 2144781 | 2.6265 | 0.09742 | 0.007 |
| refseq | species | 0.04 | adonis | Residuals | 73 | 59610720 | 816585 | 0.90258 | NA | NA |
| refseq | species | 0.04 | adonis | Total | 76 | 66045062 | 1 | NA | NA | NA |
Table R6 | Results of permutation tests at both genus species levels, and with NCBI nt and custom NCBI RefSeq databases. Beta dispersion of samples from the centroid is likely heterogeneous between each host genus, as calculated by the permutest() function in the R package vegan. Input is euclidean distances of filtered and PhILR-transformed MALT alignment OTU tables. Putative laboratory contaminants and badly-preserved samples have been removed. Test statistic is 'pseudo-F'.
| Database | Taxonomic Level | Min Support | Test | Term | Degrees of Freedom | Sum of Squares | Mean of Squares | Test Statistic | No. Permutations (permutest) | p value |
|---|---|---|---|---|---|---|---|---|---|---|
| nt | genus | 0.07 | permutest | Groups | 3 | 1066682 | 355561 | 3.3092 | 999 | 0.024 |
| nt | genus | 0.07 | permutest | Residuals | 85 | 9132865 | 107445 | NA | NA | NA |
| nt | species | 0.04 | permutest | Groups | 3 | 586810 | 195603 | 2.4151 | 999 | 0.079 |
| nt | species | 0.04 | permutest | Residuals | 85 | 6884242 | 80991 | NA | NA | NA |
| refseq | genus | 0.07 | permutest | Groups | 3 | 1325633 | 441878 | 3.0401 | 999 | 0.039 |
| refseq | genus | 0.07 | permutest | Residuals | 73 | 10610446 | 145349 | NA | NA | NA |
| refseq | species | 0.04 | permutest | Groups | 3 | 538881 | 179627 | 1.632 | 999 | 0.186 |
| refseq | species | 0.04 | permutest | Residuals | 73 | 8034883 | 110067 | NA | NA | NA |
Table R7 | Summary statistics of 100 runs of a bootstrapped PERMANOVA for each of genus and species, taxonomic levels and with NCBI nt and custom RefSeq Databases. Bootstrapping was performed on Gorilla, Pan and Homo groups only, with each run having sub-sampled each genus to 10 individuals to have equal sample size. Input data is pseudo-count and PhILR transformed MALT OTU tables, with putative laboratory contaminants and badly preserved samples removed.
| Database | Taxonomic Level | Min Support | Test | Statistic | Mean | Standard Deviation |
|---|---|---|---|---|---|---|
| nt | genus | 0.07 | Adonis | p | 0.001 | 0.001 |
| nt | genus | 0.07 | Adonis | pseudo-F | 5.228 | 1.428 |
| nt | genus | 0.07 | Adonis | RsquaredGroups | 0.275 | 0.053 |
| nt | genus | 0.07 | Adonis | RsquaredResiduals | 0.725 | 0.053 |
| nt | species | 0.04 | Adonis | p | 0.001 | 0.001 |
| nt | species | 0.04 | Adonis | pseudo-F | 6.678 | 2.525 |
| nt | species | 0.04 | Adonis | RsquaredGroups | 0.322 | 0.075 |
| nt | species | 0.04 | Adonis | RsquaredResiduals | 0.678 | 0.075 |
| refseq | genus | 0.07 | Adonis | p | 0.001 | 0.000 |
| refseq | genus | 0.07 | Adonis | pseudo-F | 6.501 | 1.648 |
| refseq | genus | 0.07 | Adonis | RsquaredGroups | 0.321 | 0.054 |
| refseq | genus | 0.07 | Adonis | RsquaredResiduals | 0.679 | 0.054 |
| refseq | species | 0.04 | Adonis | p | 0.001 | 0.000 |
| refseq | species | 0.04 | Adonis | pseudo-F | 6.886 | 1.947 |
| refseq | species | 0.04 | Adonis | RsquaredGroups | 0.332 | 0.059 |
| refseq | species | 0.04 | Adonis | RsquaredResiduals | 0.668 | 0.059 |
The R script for summarising the results across all runs is also
017-PhILR_PCoA_summaries.R, and the output files for each combination are in
00-documentation under philr_permanova_*_summary.tsv.
Overall we found that despite some overlap (as seen in the PCoA analysis, the calculus microbiomes of each host genus could be considered statistically distinct.
To visualise possible drivers of similarity and differences between the different host genera, we applied hierarchical clustering on the contaminant and preservation filtered MALT OTU tables, which are then displayed as heatmaps.
This is performed in the R notebook and script version
02-scripts.backup/045-Compositional_Heatmaps.R(md)
The procedure again follows CoDa principles by performing CLR transformation of the OTU matrix (rather than PhILR as so to retain information on the actual taxa classes that are driving differences), upon which unsupervised clustering of host taxa and microbial taxa is applied. The clustering algorithm actually used is selected automatically within the script. Finally, a heatmap representation of the clustering is generated.
The script version allows additional filtering as above for the Principle Coordinate and PERMANOVA analyses (database, taxonomic levels, with/without sources, with/without controls, with/without bad samples (+ bad sample removal method option)) and also additional taxon filtering, zero-replacement method, additional min. support filtering. Finally, an additional filter was included: a prevalence filter (i.e. a taxon is only kept if it is in n number of individuals across the dataset).
We modified the above parameters to find the combination that resulted in the most robust overall bootstrap support in the deepest nodes (i.e. the ones we are most interested in this study - splits between host genus level clades)
## Additional min. support filtering, no genus filtering
Rscript 02-scripts.backup/045-Compositional_Heatmaps_20190806_script.Rmd nt species noSources noControls out withinvariation none pseudo 4 5The sample clustering with no taxon filtering, additional min. support of 0.04, and prevalence filtering set to 5 was selected. This was based on it having generally the highest bootstrap values in the internal nodes, and the phylogeny showing 'cleanest' clustering of individuals of the same host genus falling together. Interpretation of these heatmaps is described in the main publication.
To aid interpretation, phenotypic data of the taxa displayed in the heatmap was
performed via 02-scripts.backup/046-bacdive_searcher.R, and added manually to
the heatmap plots using Inkscape, which was recorded in the file
00-documentation.backup/99-Heatmap_ManualBlockDescriptions_alltaxa_minsupportmultiplier4_minprevalence4_databasent_metadata.tsv
The final figures for both databases can be seen collated in
06-additional_data_files under Data R20 or individual files in
04-analysis/screening/compositional_heatmaps.backup.
In general we observed Gorilla and Alouatta displayed more diversity of aerobic taxa, whereas Pan had lower diversity but consisted primarily of anaerobic and late colonising taxa.
We also checked whether the hierarchical clustering results was affected by the zero replacement model, with the same script and otherwise the same settings.
Rscript 02-scripts.backup/045-Compositional_Heatmaps_20190806_script.Rmd nt species noSources noControls out withinvariation none czm 4 5
Comparing the zero replacement methods showed no difference between clustering. There were only cosmetic tree topology changes with clade rotation, i.e. no structural changes. The output is saved as in the same directory at above.
To confirm that the species corresponding to the grouping observed in the
hierarchical clustering, we ran Indicator Analysis to find species that are
'indicative' of certain host genera combinations. This was performed with the R
notebook 02-scripts.backup/020-Indicator_analysis_20190808.Rmd.
The results can be seen can be seen in 06-additional_data_files under Data
R21 or in 04-analysis/screening/indicspecies.backup, with discussion in
the main publication.
To revisit the question and results posed by Weyrich et al. (2017) Nature,
regarding clustering of the calculus microbiomes individuals by subsistence
strategy, we similarly performed PCoA, PERMANOVA, hierarchical clustering on
Neanderthals and ancient humans - however in this case with a more balanced
sampling strategy. This was performed in the notebook
02-scripts.backup/017-PERMANOVA_HomoOnly_Dietary_20190916.Rmd and
corresponding script version.
The results are saved in 04-analysis.backup/screening/philr_dietary.backup/
and seen in Figures R22 and R23.
Figure R22 | Principal coordinate analysis of different Homo calculus microbiomes, comparing different lifestyles and regions. a we do not observe clustering of calculus microbiomes of individuals from Homo by broad dietary differences, by prokaryotic OTUs at genus level, as originally reported by Weyrich et al. 6. b we do not observe a clear regional difference between microbiomes that may indicate preservational biases. Input is a genus level PhILR transformed OTU table without sources and controls, and low preservation samples removed. Low abundant taxa are removed if under 0.01% of overall alignments ('min. support'), and putative laboratory contaminants removed as per the decontam R package.
Figure R23 | Hierarchical clustering of different Homo calculus microbiomes, comparing different lifestyles and regions. We do not observe clustering of calculus microbiomes of individuals from Homo by broad dietary differences. Input is a genus level PhILR transformed OTU table without sources and controls, and low preservation samples removed. Clustering was performed with the average-linkage algorithm. Low abundant taxa are removed if under 0.01% of overall alignments ('min. support'), and putative laboratory contaminants removed as per the decontam R package.
More discussion of the results can be seen in the main publication, however overall we observe that we are unable to distinguish between each 'subsistence' strategy given other factors such as age or region. Much more balanced and controlled sampling is required to address this question to recover whether diet indeed effects the taxonomic compositional of oral microbiomes.
Given the overlap between each host genus as identified above in the principle coordinate analysis, we also wished to find microbiota taxa that are potentially present across various combinations of the host genera.
For this we ran a core microbiome estimation analysis via intersection of
presence/absence of different OTUs, as shown in the R notebook
02-scripts.backup/018-CoreMicrobiome_20190902.Rmd. A script version of the
notebook is also provided under
02-scripts.backup/018-CoreMicrobiome_20190902_script.R.
See the notebook and main publication for more details in how the intersection procedure was constructed (to control for biological and preservational variation), however Figure R24 shows a schematic for this.
Figure R24 | Schematic of parameters used for selecting taxa considered to be core to a host genus population and host genus population themselves. Requiring half of individuals of a population (to be core to a population) allows for inter-individual biological variability and preservation variability. Requiring two-thirds of the populations of a host genus (to be core to a host genus), ensures a particular taxon is core in multiple populations or subspecies and is not unique to a single population (which may also reflect preservational or curational backgrounds). Alouatta is exempt from the population level parameter due to the inclusion of only a single population.
We found that requiring a taxon to be present in 50% of a population individuals, and 66% of host populations provided robustness against preservational and biological variability, while having enough individuals/populations for corroboration that a taxon could be considered 'core'.
Visual inspection of the initial results of the intersection analysis with the number of individual parameters selected above, showed incorporation of well-known environmental contaminatant taxa still occurred.
We therefore took a conservative approach and explored further optimal minimum support values (extrapolated from above) that removed well-known environmental taxa but did not equally remove well-known oral-specific taxa.
To do this we re-ran with a variant of the notebook in the section above, but with different minimum support multipliers.
Code for this procedure can be seen in
02-scripts.backup/018-CoreMicrobiome_ParameterTesting_20190902.Rmd), we
collated the results from each database and taxonomic level into a single set
of results using the R script
02-scripts.backup/018-CoreMicrobiome_summaries_20190902.R. This collected set
of results can be seen in
00-documentation.backup/23-intersection_proktaxapassingthresholdsstats_20190211.tsv
and
00-documentation.backup/24-intersection_proktaxapassingthresholdstaxalist_20190211.tsv
Figure R25 shows the results of increasing the minimum support filter at genus and species level for both databases.
Figure R25 | Alluvial diagram showing effects of increasing the minimum abundance threshold to the MALT OTU table-based core microbiome calculations. Increasing from 0.04% to 0.07% shows minimal changes in combination assignment. Comparisons are between the nt (top) and RefSeq (bottom) databases, and at genus (left) and species (right) taxonomic levels. Stacked bars represent the number of taxa to each combination, and alluviums represent the assignment of a given taxon between each minimum support threshold. Plots created using the ggalluvial R package 273, with input data as MALT aligned and MEGAN exported OTU tables excluding putative laboratory contaminants, badly preserved samples, and taxa with minimum support values < 0.07% (genus level) and < 0.04% (species level).
Setting a 0.07% minimum support value at genus level and 0.04% for species was sufficient to remove all well-known environmental or contaminant taxa while retaining well-known oral taxa. Furthermore, these parameters generally removed remaining laboratory contaminants being considered core to a 'controls + host genus' combination.
The raw data for the min. support permutation comparison can be seen under
06-additional_data_files in Data R23.
During sample-preservation filtering above, one of the Gorilla populations was left with a single individual - which could cause a 'false positive' support for a core taxa, if the taxa wasn't actually present in most individuals of that population.
We therefore looked a the effect of removing the single individual
population for Gorillas, with the script version of the core microbiome notebook,
(02-scripts.backup/018-CoreMicrobiome_ParameterTesting_20190902.R)
and permutating whether any populations with a single individual were dropped
or not.
## Final being minSupport of X, 0.5 inds of population, and 66 pops of genus,
## min. support 0.7 for genus and 0.4 for species, and testing whether dropping
## single individual populations makes a difference.
for i in 4 7; do
Rscript 02-scripts.backup/018-CoreMicrobiome_20190429_script.R "nt" "$i" 0.5 0.66 F
Rscript 02-scripts.backup/018-CoreMicrobiome_20190429_script.R "nt" "$i" 0.5 0.66 T
done
for i in 4 7; do
Rscript 02-scripts.backup/018-CoreMicrobiome_20190429_script.R "refseq" "$i" 0.5 0.66 F
Rscript 02-scripts.backup/018-CoreMicrobiome_20190429_script.R "refseq" "$i" 0.5 0.66 T
done
Figure R26 | Alluvial diagram showing effects of dropping and retaining a single-individual Gorilla population in core microbiome calculations at genus and species taxonomic levels. Dropping the single-individual population results in minor combination assignments, mostly taxa being assigned to being core to the compositionally similar Alouatta combinations. Stacked bars represent the number of taxa to each combination, and alluviums represent the assignment of a given taxon between dataset. Plots created using the ggalluvial R package, with input as MALT NCBI nt aligned and MEGAN exported OTU tables with putative laboratory contaminants, badly preserved samples, and taxa with minimum support values < 0.07% (genus level) and < 0.04% (species level) removed.
The raw data for the comparison of excluding single individual populations can
be seen under06-additional_data_files in Data R24.
Individual visualisations and results for each parameter run can be seen in
04-analysis/screening/presenceabsence_intersection.backup/.
Discussion of these results can be seen in the main publication. In general, only minor differences were observed suggesting the Gorilla core was generally robust.
To assess the robusticity of our overall core microbiome calculations, we additionally performed subsampling and bootstrapping analysis of each host genera. By subsampling and randomly selecting the individuals of each host genera, we aimed to see how often a given microbial taxon was considered 'core' to a given host-genus combination.
The code can be seen under
02-scripts/018-CoreMicrobiome_20200908_complex_bootstrapping.Rmd.
We ran the notebook for each database and microbial taxa level (at the corresponding min. support filters), both with and without controls. We observed that for the main 'anthropoid' core taxa (the term here used for Hominids and _Alouatta), in most cases lower bootstrap values occured due to re-assignment with the 'anthropoid+control' combination. We reasoned we could perform the bootstrapping without blanks given the low-biomass nature of them making picking up oral taxa (a common contaminant) is likely as the number of taxa are already low. i.e. tail filters (such as min. support) would not remove these oral axa even if the number of reads of reads a very low to negliable, as the min. support tail applies on a per-sample basis - in other words, the behaviour of the min. support filter has less an effect in removing contaminants on controls compared to samples. As we wished to check robusticity of the core taxa of actual samples, it was OK to exclude these in this particular bootstrapping test. We therefore re-ran the core microbiome calculations with 1000 subsampling replicates (with replacement), both including and excluding controls.
The outcome of these bootstrapping results can be seen in
04-analysis/screening/presenceabsence_intersection/99-coremicrobiome_presenceabsence_pergroup_bootstrapped_complete_*,
with combinations of which database was used (nt or refseq), which microbial
taxonomic level used (species or genus, with corresponding minsupport values).
Two files are created for each one with all possible combinations encountred for
each taxon (_complete_), and another with just the most commonly found
combination (_tophits_).
A comparison between the nt bootstrapping runs with and without controls being
included can be seen in
04-analysis/screening/presenceabsence_intersection/99-coremicrobiome_presenceabsence_pergroup_bootstrapped_complete_nt_speciesgenus_withwithoutcontrols_comparison.tsv.
A condensed version of this comparison can be seen in Table R8.
Table R8 | Comparison of core micrbiome bootstrappig results when including and excluding controls Bootstrapping was performed on all host groups (and controls), with 1000 replicates. FALSE/TRUE column headers refer to exclusion or inclusion of controls respectively. Bold indicate values equal to or exceeding 75%. Note genus level taxa have min. support values of 0.07 whereas species level taxa have a min. support value of 0.04.
| Combination | Taxon | FALSE | TRUE |
|---|---|---|---|
| Alouatta:Gorilla:Homo | Eikenella | 92.7 | 73.7 |
| Alouatta:Gorilla:Homo | Neisseria | 87.1 | 90.9 |
| Alouatta:Gorilla:Homo | Aggregatibacter | 99.8 | 94.6 |
| Alouatta:Gorilla:Pan:Homo | Corynebacterium | 100 | 87.4 |
| Alouatta:Gorilla:Pan:Homo | Prevotella | 91.5 | 80.4 |
| Alouatta:Gorilla:Pan:Homo | Ottowia | 95.5 | 90.4 |
| Alouatta:Gorilla:Pan:Homo | Campylobacter | 99.3 | 98.7 |
| Alouatta:Gorilla:Pan:Homo | Selenomonas | 92.4 | 70 |
| Alouatta:Gorilla:Pan:Homo | Capnocytophaga | 71.6 | 99.9 |
| Alouatta:Gorilla:Pan:Homo | Fusobacterium | 100 | 75 |
| Alouatta:Gorilla:Pan:Homo | Pseudopropionibacterium | 100 | 92.2 |
| Alouatta:Gorilla:Pan:Homo | Actinomyces | 100 | 52.3 |
| Alouatta:Gorilla:Pan:Homo | Streptococcus | 100 | 31.2 |
| Alouatta:Homo | Leptotrichia | 84 | 83.2 |
| Alouatta:Pan:Homo | Gemella_sp._oral_taxon_928 | 91.8 | 93.4 |
| Gorilla:Pan:Homo | Fretibacterium | 84.5 | 31.4 |
| Gorilla:Pan:Homo | Tannerella | 34 | 33.5 |
| Gorilla:Pan:Homo | Olsenella | 35 | 58.6 |
| Gorilla:Pan:Homo | Anaerolineaceae_bacterium_oral_taxon_439 | 76.7 | 84.2 |
| Homo | Veillonella | 8.7 | 10.9 |
| Pan:Homo | Parvimonas | 24 | 25.7 |
| Pan:Homo | Mogibacterium | 68.5 | 68.2 |
| Pan:Homo | Porphyromonas | 13.9 | 16.1 |
| Pan:Homo | Treponema | 22.8 | 24.1 |
| Pan:Homo | Filifactor_alocis | 76.7 | 77.2 |
| Pan:Homo | Desulfomicrobium | 100 | 100 |
When considering a bootstrap cut off of 75% and excluding controls, the majority of the Hominid and Alouatta core taxa can be considered to be found robust when randomly subsampling (with replacement), with only Capnocytophaga falling below this at 71.6. For this particular taxon, alternative combinations that appeared during bootstrapping replicates were the following: Alouatta:Gorilla:Homo, Alouatta:Homo, Alouatta:Pan:Homo. That there are multiple different combinations occuring, rather than a consistent single alternative, this may suggest this taxon may still be prevalent in all host genera just has fewer read abundances and therefore detection maybe more stochastic depending on each individual's biofilm make up. A similar inteperretaion can also be applied to the other lower values such as Tannerella, Olsenella, Porphyromonas and Treponema, who mostly display 3 or more alternative combinations rather than a single alternative; all of which are different combinations of all the host genera (i.e. not consistently with one or two host genera), suggesting common presence across all host genera but at a lower prevalence - possibly again due to low read levels. Given the three 'red complex' taxa, are mostly known to be more commonly found in more mature calculus deposits, the lower bootstrap value may represent an artfacet of the maturity of the sampled calculus from these individuals (i.e. only when multiple individuals with mature deposits are including in subsampling does the core microbiome of that taxa is the taxon assigned to wider combinations)
Inclusion of controls sees a reduction in the bootstraps in most cases, at varying levels. This is somewhat to be expected due to oral taxa being common contaminants in lab reagents, and min. support values (i.e. tail trimming) having a less of a effect (i.e. the fewer overall reads sequenced due to the low biomass nature of controls meaning fewer true low-level contaminants taxa are removed because the overall number of reads is low). Across all the well supported bootstrap values. Most hominid/Alouatta core microbiome assignments are reduced due to being assigned to a control combination. In particular, Streptococus and Actinomyces sees the largest drops, however these are highly diverse genera and therefore non-oral species under these genera are likely to be present in skin and other modern human contaminants sources. This therefore does not indicate these are not authentic oral taxa given other lines of evidence at species level as shown in other analysis.
In the resulting list of taxa that were a part of the core of each combination
of host taxa, we observed that Mycobacterium was able to pass our minimum
support despite being a common soil contaminant. We investigated this further
in an extension of the original Core Microbiome calculation script which is
under 02-scripts.backup as
099-CoreMicrobiome_ParameterTesting_and_MycobacteriumInvestigation_20190902.Rmd
Figure R27 | Alignment distribution and prevalence of Mycobacterium across well-preserved calculus microbiome samples in this study. Approximate 'abundance' is consistent across calculus samples, however most prevalent taxa are likely well-known environmental taxa. a summary of alignments of well preserved samples and sources to Mycobacterium species. b number of individuals that all Mycobacterium species identified in the dataset are found in. Input is from MALT NCBI nt database OTU table at species level, excluding putative laboratory contaminants and badly preserved samples.
We noted that the taxa being picked up with MALT were indeed highly prevelent soil contaminants that are typically waterborne, and we therefore concluded this was likely derived from taphonomic causes.
To assist in the interpretation of results, we wanted to create a visualisation to efficiently summarise the number of taxa associated with the core of each host combination.
Due to the large number of categories, the typically used Venn diagrams become
difficult to understand due to the inclusion of many layers of overlapping
combinations. Instead, we summarised the number of taxa in each host via an
'UpSet' plot. Code for this can also be seen in the noteboom
02-scripts.backup/018-CoreMicrobiome_ParameterTesting_20190902.Rmd.
Figure R28 | UpSet plot showing the number of taxa shared across each host genus combination for NCBI nt (top) and custom NCBI RefSeq (bottom) and genus (left) and species (right). Note that for the custom RefSeq database plots, a control group as a 'core' microbiome is displayed as at the corresponding minimum support value. However these taxa remain unique to the control samples only, which does not exist at the same threshold for the nt database. Plots are generated from MALT aligned and MEGAN exported OTU tables to each database; filtered for putative laboratory contaminants, badly preserved samples and a minimum support value for microbial taxa of 0.7 (genus level) and 0.4 (species level). Taxa are considered core to a host genus if taxon is present in 50% of individuals of each population, and >= 66% of the populations to a given host. Note the the difference between this figure and Figure 2 of the main manuscript is the retention here of Mycobacterium, which was excluded post-hoc (and thus excluded from Figure 2), and from subsequent the downstream core microbiome analysis, given that section R10.1.5 shows it is a pervasive likely contaminant.
The final list of taxa at both species and genus level that we estimated as
being core to each host group and combinations thereof, can also be seen in
06-additional_data_files under Data R22. Discussion of the core microbiomes
can be seen in the main text.
Additionally, we compared the core assignments between the two databases. These
comparisons were generated with
02-scripts/18-CoreMicrobiome_database_Comparison, and the outcome can be
seen in 06-additional_data_files under Data R25.
Overall we found that core genera and species did exist across all host genera in this study. These taxa span all layers of the known biofilm structure in modern human calculus, suggesting a highly conserved structural core across deep time. See main publication for further discussion.
To further verify the authenticity of the ancient nature of the core microbiome assignments in the calculus samples - we also ran MaltExtract on a subset of the core taxa to check for damage patterns and short fragment lengths characteristic of ancient DNA.
We took the species level 'core' of the Hominids/Alouatta, Hominids and Homininis based on the MALT nt database (as estimated above - i.e. with a min. support value of 0.04, requiring a taxon being in 50% of each population having a taxon to be core to the population, 66% of the populations to be core to the genus, and retained single individual populations).
This list of taxa was then given to MaltExtract as follows,
MaltExtract \
--destackingOff \
-f def_anc \
-i 04-analysis/screening/maltExtract/input/all/ECO*rma6 \
-o 04-analysis/screening/maltExtract/output/test/ \
-p 32 \
-r RMA_Extractor_Resources \
-t 04-analysis/screening/maltExtract/taxon_lists/08b-coremicrobiome_presenceabsence_upsettable_allsoftware_maltdbnt_0.04_fracinds0.5_fracpops0.66_singleindpopsdroppedF_hostgenus_species_AnthropoidsHominidaeHoiminini_20190509.tsv \
-v
See above for the raw MaltExtract results - as this data has been uploaded to the Github repository for MEx-IPA.
We next wanted to test whether the phylogenies of specific oral taxa also 'mimic' the phylogenetic histories of the host. A common problem from genome construction from metagenomic sources - particularly with microbiome data - is cross-mapping from related strains and species. This makes genotyping difficult when dealing with the low coverage data, because the confidence in the SNP calling is very low as variants of a position can come from multiple 'conflicting' sources.
As a strategy to try and reduce cross-mapping, I came up with the idea to map the production dataset to a 'super-reference' of all relevant species of a genus (including the target species of interest), and then only genotype on the section of the super-reference including the target species of interest. The predicted effect would be reads from off-target genomes would be attracted to the original related strains/species and thus would not be present on the reference of interest itself.
For optimal phylogenetic reconstruction, high coverage genomes are generally preferred to improve confidence in genotyping. The screening dataset generally yields low coverage genomes, and we wanted to work out which samples would provide the best chance of producing multiple higher-coverage genomes of species of interest.
To calculate this, we mapped to a range of taxa of interest (either from observations in the dataset or from broad literature review). We selected the following species - downloaded the reference or representative strains from NCBI, and indexed as above.
The Initial species that were selected and downloaded are:
- Pseudopropionibacterium propionicum
- Treponema socranskii (only scaffolds)
- Rothia dentocariosa
- Desulfobulbus sp. oral taxon 041 (<- only contigs)
- For this selected the sp. oral taxon 041 assembly with the largest assembly size, Desulfobulbus sp. oral taxon 041 str. Dsb1-5.
- Downloaded from the Genbank FTP server the contigs from here: ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCA/000/403/865/GCA_000403865.1_Dsb1-5
- Fusobacterium nucleatum
- Aggregatibacter aphrophilus
- Streptococcus gordonii
- Treponema denticola
- Tannerella forsythia
- Treponema denticola
We then mapped all our samples to the references listed above with EAGER, with the parameters below.
Organism: Bacteria/Other
Age: Ancient
Treated Data: non-UDG
Pairment: Single End
Input is already concatenated (skip merging): Y
Reference: [listed above]
Name of mitocondrial chromosome: [none]
FastQC: Off
AdapterRemoval: Off
Mapping: BWA
Seedlength: 32
Max # diff: 0.1
Qualityfilter: 37
Filter unmapped: On
Extract Mapped/Unmapped: Off
Complexity Estimation: Off
Remove Duplicates: DeDup
Treat all reads as merged: On
Damage Calculation: Damageprofiler
SNP Calling: Unified Genotyper
Emit All Sites: On
CleanUp: On
Create Report: On
⚠️ EAGER results files are not included here due to large size
We initially tried running PreSeq, but (un)fortunately the library complexity was too high and not enough duplicates were found in most of the mappings to yield enough information for extrapolation of additional sequencing required.
Instead, we did a linear estimation in an spreadsheet. For this we took the following steps.
- Multiplying the current number of reads and depth coverage until target depth coverage is reached (5x)
- Remove any mapping that required more >100 million reads
- Remove any mapping with a 'cluster factor' (reads before mapped deduplication / mapped reads after deduplication) above 1.2 - suggesting lower complexity library
- Select any individual with at least 3 mappings retained above above filtering (if a host genus did not have enough samples, we went with the next highest number of mappings), with a target sample size of 3 individuals per host group.
Extracts of the selected samples were sent for UDG treatment and deep sequencing for the majority of downstream phylogenetic and genome-level functional analysis analysis.
Additional samples were also included if previously deep sequenced for other contexts
Now with the deep sequenced subset of individuals, we then decided to select taxa of the Hominid/Alouatta core microbiome we calculated above. We made the assumption these taxa would be more likely to yield enough mappings across all host genera in this study sufficient for phylogenetic analysis across the Hominid evolutionary history.
To assess whether we could reduce the number of multi-allelic positions caused
by cross-mapping, we first generated a super-reference for each genus,
as described in the the notebook
02-scripts.backup/99-phylogeny_reference_genome_collection.Rmd.
This notebook downloads the NCBI Genome assembly reports, performs filtering based on sequencing level, quality, whether it is representative or not - and selects one strain for species within the genus. Multi-chromosome or contig assemblies were also collapsed into single entry.
Within the notebook we also performed more manual filtering of isolation source to remove species that are very unlikely derived from the human body or in contact with archaeological samples (such as activated sludge).
The outcome of these filtering steps can be seen in the files
00-documentation.backup/18-Core_Microbiome_AssemblyDownload_*, with the final
files used for downloading ending with "*filtered". These files were
downloaded as above with wget.
Pseudopropionibacterium is generated in a different manner due to recent clade renaming, but the desire to retain common skin taxa which now have new genus names (see Scholz and Kilian 2016)
You can alternatively see a list of all genomes downloaded for each
super-reference in 06-additional_data_files under Data R26.
We converted the FASTA headers to a format suitable for samtools and
bedtools (i.e. replace entries with entire species names, rather than
'chromosome' IDs) with
02-scripts.backup/099-fasta_header_replacer.sh <FASTA>.
Then, for certain aspects of downstream processing (in particular
MultiVCFAnalyzer), we needed to convert the multiple FASTA files into a single
one, with FASTA headers removed. For this we used the script
02-scripts.backup/099-collapse_fastas.sh, which combines them, but exports
coordinates (also in bed format) indicating where each species FASTA entry is
present, in the combined super-reference FASTA.
FASTAs were indexed as above
⚠️ Reference files and indices are not included due to large size
The production dataset was then mapped to each of the core genus super-references with EAGER, with the following settings.
Organism: Bacteria/Other
Age: Ancient
Treated Data: UDG
Pairment: Single End
Input is already concatenated (skip merging): Y
Reference: [listed above]
Name of mitochondrial chromosome: [none]
FastQC: Off
AdapterRemoval: Off
Mapping: BWA
Seedlength: 32
Max # diff: 0.1
Qualityfilter: 0
Filter unmapped: On
Extract Mapped/Unmapped: Off
Complexity Estimation: Off
Remove Duplicates: DeDup
Treat all reads as merged: On
Damage Calculation: On
SNP Calling: On
Emit All Sites: Y
CleanUp: On
Create Report: On
The settings above in table format can be seen in 06-additional_data_files
under Data R27.
⚠️ The EAGER mapping results files are not provided here due to their large size, other than the ReportTable files which is stored in the directory.04-analysis/deep/eager/superreference_mapping/output
To generate breadth and depth statistics for each species reference (see below)
in the super-reference we used bedtools, using the BED coordinates as
provided by the 02-scripts.backup/099-collapse_fastas.sh for each species.
bedtools coverage -a 01-data/genomes/"$genus"/collapsed_"$genus"_superreference.bed -b "$BAM" | pigz -p 1 > "$BAM".bedtoolsbreadth.tsv.gz
bedtools coverage -a 01-data/genomes/"$genus"/collapsed_"$genus"_superreference.bed -b "$BAM" -mean | pigz -p 1 > "$BAM".bedtoolsdepth.tsv.gzThis was adapted from a SLURM array script using
findand should be adapted accordingly to your own system so that thaBAMvariable covers all mapping deduplicated BAM files for each individual and super-reference
Output results for this statistics can also be see under
04-analysis/deep/eager/superreference_mapping/output or collated in
06-additional_data_files under Data R28.
While we now had a super-reference mapping, we needed to compare the whether genotyping on the target-species region of a super-reference reduced the number of multi-allelic SNPs when compared to genotyping with a single reference genome
For this, we needed a single genome to compare to, however the presence/absence based core microbiome analysis nor heatmaps above did not provide much information on the most optimal species to select for each core genus for comparison (i.e. should we select Streptococcus sanguinis or Streptococcus gordonii? Which one has the higher coverage, in the most samples across our host genera).
To select the best 'target' species to compared to, we used the depth and
coverage statistics for each species in each super-reference to calculate a
variety of metrics, across which we used to select the best fitting
species for the two requirements above. This is described in
02-scripts.backup/031-superreferencemapped_genotyping_stats.Rmd.
We did this via both 'visual inspection' of plots, and tried an 'automated' approach by selecting which taxon was top of the highest number of the 5 metrics used (see notebook for both procedures).
The selection based on the different metrics performed manually and via
an automated system was compared in 06-additional_data_files under
Data R29. The list below in Table R9 showed high concordance with the visual
inspection, and therefore the visual taxon selection was used only when
there was not a clear 'winner' from the automated selection (e.g. Selenomonas)
The final list of selected taxa via visual inspection were as follows
- Actinomyces - Actinomyces_dentalis_DSM_19115
- Campylobacter - Campylobacter_gracilis
- Capnocytophaga - Capnocytophaga_gingivalis_ATCC_33624
- Corynebacterium - Corynebacterium_matruchotii_ATCC_14266
- Fretibacterium - Fretibacterium_fastidiosum
- Fusobacterium - Fusobacterium_hwasookii_ChDC_F206
- Olsenella - Olsenella_sp_oral_taxon_807
- Ottowia - Ottowia_sp_oral_taxon_894
- Porphyromonas - Porphyromonas_gingivalis_ATCC_33277
- Prevotella - Prevotella_loescheii_DSM_19665_=JCM_12249=_ATCC_15930
- Pseudopropionibacterium - Pseudopropionibacterium_propionicum_F0230a
- Selenomonas - Selenomonas_sp_F0473
- Streptococcus - Streptococcus_sanguinis_SK36
- Tannerella - Tannerella_forsythia_92A2
- Treponema - Treponema_socranskii_subsp_paredis_ATCC_35535
Output from the automated species selection can be seen in
04-analysis/deep/competitive_mapping.backup/species_selection. This is
also summarised in Table R9, and the majority call under the 'Selected'
column.
Table R9 | Results of ‘automated’ super-reference species selection for downstream phylogenetic analysis. Production dataset was mapped for each genus with EAGER to a combined reference of all species of the calculus microbiome core genome calculated above. Metrics were number of reads, breadth coverage, depth coverage, competitive mapping score, percentage of species reads over all genus reads. Species were then filtered those that had a number metrics that the species exceeded the genus mean plus standard deviation of the metric was more than 1. In cases of ties, either a named species or more complete genome reconstruction selected. In cases where unnamed species are the top candidate, either the next best taxon with an official name or where the isolation source was ‘oral cavity’ were selected.
| Genus | Species | Metrics Passed | Selected |
|---|---|---|---|
| Actinomyces | Actinomyces dentalis DSM 19115 | 5 | TRUE |
| Campylobacter | Campylobacter sp. AAUH-44UCsig-a | 3 | FALSE |
| Campylobacter | Campylobacter gracilis | 2 | TRUE |
| Capnocytophaga | Capnocytophaga gingivalis ATCC 33624 | 5 | TRUE |
| Corynebacterium | Corynebacterium matruchotii ATCC 14266 | 4 | TRUE |
| Corynebacterium | Corynebacterium sp. | 3 | FALSE |
| Corynebacterium | Corynebacterium durum F0235 | 2 | FALSE |
| Fretibacterium | Fretibacterium fastidiosum | 4 | TRUE |
| Fusobacterium | Fusobacterium hwasookii ChDC F206 | 2 | TRUE |
| Olsenella | Olsenella sp. oral taxon 807 | 5 | TRUE |
| Ottowia | Ottowia sp. Marseille-P4747 | 4 | FALSE |
| Ottowia | Ottowia sp. oral taxon 894 | 3 | TRUE |
| Porphyromonas | Porphyromonas gingivalis ATCC 33277 | 4 | TRUE |
| Prevotella | Prevotella loescheii DSM 19665 = JCM 12249 = ATCC 15930 | 2 | TRUE |
| Prevotella | Prevotella sp. oral taxon 472 str F0295 | 2 | FALSE |
| Prevotella | Prevotella sp. S7 MS 2 | 2 | FALSE |
| Pseudopropionibacterium | Pseudopropionibacterium propionicum F0230a | 4 | TRUE |
| Selenomonas | Selenomonas sp. F0473 | 2 | TRUE |
| Selenomonas | Selenomonas sp. oral taxon 137 str F0430 | 2 | FALSE |
| Streptococcus | Streptococcus sanguinis SK36 | 4 | TRUE |
| Streptococcus | Streptococcus sp. AS14 | 4 | FALSE |
| Tannerella | Tannerella forsythia 92A2 | 5 | TRUE |
| Treponema | Treponema socranskii subsp. paredis ATCC 35535 | 5 | TRUE |
The reference genomes of the selected taxa were then copied from the
super-reference downloaded files,
and multiple chromosomes collapsed as again described in
02-scripts.backup/99-phylogeny_reference_genome_collection.Rmd.
EAGER was run again with the same settings for the Super-reference mapping, but with the single representative genome taxa FASTAs instead.
Summary plots of the single genome mappings can be seen under
02-scripts.backup/099-SingleGenome_MappingStatistics_Summary.Rmd. Corresponding
EAGER statistics can be seen in 06-additional_data_files under Data R32.
Summary statistics comparing mapping to a super-reference and single reference
genome can be seen in 06-additional_data_files under Data R30.
⚠️ The reference genome files are not provided here due to the large size
Figure R29 | Comparison mapping statistics of deep sequenced calculus microbiomes to single species representatives of core calculus microbiome genera. Despite deep sequencing, mean fold coverage remains low - albeit with low cluster factor suggesting deeper sequencing will result in higher coverages. a Distributions of mean fold coverage. b Distributions of cluster factor. Mappings are production dataset calculus data, mapped to a single representative reference genomes of core hominid/Alouatta calculus microbiome. Post-deduplication mean fold coverage and cluster factors values are as reported by EAGER results table.
As the main target of this comparison of mapping strategies was to see if we could reduce the level of cross-mapping that can cause problematic genotyping, we needed a way of assessing the level of multi-allelic positions occur in each mapping.
For this we used MultiVCFAnalyzer, which, when given a GATK UnifiedGenotyper VCF file where the reference ploidy was ('fakely') set to 2, will report the fraction of reads that hold an allele in 'heterozygous' sites (i.e. positions where there is a possible reference and a possible alternative allele - which is not expected in haploid bacteria).
We ran MultiVCFAnalyzer as follows for each reference species of the two mapping strategies (in the case of the super-reference mapping, of the 'extracted' region of interest).
java -Xmx32g -jar MultiVCFanalyzer_0-87.jar \
NA \
"$FASTA" \
NA \
<OUTDIR> \
T \
30 \
2 \
0.9 \
0.1 \
NA \
<VCF_1> \
<VCF_2> \
<VCF_3>
We set T to turn on saving of the allele frequencies in the outputted SNP
Table, minimum coverage of 2 (to ensure at least two independent reads support
a call), a minimum fraction of reads required to have a base to be considered
a single-allelic reference or SNP call as 0.9, and a minimum of 0.1 (but below 0.9) to be considered heterozygous.
For super-reference mappings, we still needed to retrieve the statistics for just the positions representing the target species of interest. We used the following script to extract these from the MultiVCFAnalyzer SNP table output.
for i in 04-analysis/deep/multivcfanalyzer/initial_single_genome/output/*; do
species="$(basename $i)"
genus="$(echo $species | cut -d_ -f 1)"
Rscript 02-scripts.backup/036-generate_multiVCFanalyzer_subset_statistics_20190322.R \
04-analysis/deep/multivcfanalyzer/superreference_mapping/output/"$genus"/ \
04-analysis/deep/eager/superreference_mapping/references/"$genus"/collapsed_"$genus"_superreference.bed \
"$species"
doneOutput files can be seen under
04-analysis/deep/multivcfanalyzer/superreference_mapping/output/
⚠️ Only the snpStatistics files are provided here due to the large size of the other MultiVCFAnalyzer output files.
To compare the level of heterozygosity as reported in MultiVCFAnalyzer between
the two mapping strategies, we created the following R notebook:
02-scripts.backup/032-multibasesites_mappingstrategycomparison_20190528.Rmd.
A summary table of percentage of multi-allelic SNPs when running
MultiVCFAnalyzer using both mapping methods can be seen in
06-additional_data_files under Data R30. Graphical comparison can be seen
below in Figure R62.
Figure R62 | Comparison of the number of multi-allelic single nucleotide variants (SNPs) identified when using a single representative genome mapping strategy versus a multi-reference genome (super-reference) mapping approach. Analysis was performed on selected abundant and prevalent species representatives of core Hominid/Alouatta genera, using the production dataset. Multi-allelic SNPs are sites called with more than the expected 1 allele (given haploid bacteria) – indicating cross mapping from other strains or related species. To reduce the occurrence of these and improve phylogenetic power, mapping to multiple species of a genera (vs. the single representative) to attract away mis-mapping reads was attempted. Arrows indicate direction of change in the percentage of multi-allelic sites between single genome (grey circle outline) and super-reference (black circle outline) mapping strategies. The expected reduction in the percentage of multi-allelic SNPs (from right to left on the x-axis) between the two strategies is not observed to consistently occur and with minor effect. Furthermore, in most cases when a reduction occurs, these samples have already very few SNPs (size circles) usable for phylogenetic analysis. Statistics are summarized from the ‘SNP statistics’ file from the output of MultiVCFAnalyzer with a ‘homozygous’ threshold of 0.9, a ‘heterozygous’ threshold of 0.1, and a minimum coverage threshold of 2.
Note: the figure ID is out of order due to missing upload during initial writing of this walk-through.
From this script and in the figure above, we observed that the super-reference mapping strategy did not work very often at reducing the number of multi-allelic SNPs, and further often resulted in a large decrease in the number of positions overall on the reference itself - therefore reducing the phylogenetically-informative number of sites (see many publication for more discussion). We therefore selected the single representative genome mappings for downstream analysis to ensure maximal information for relationship calculations.
Additionally, in the notebook we also show that we identified the most common fraction of a majority allele in multi-allelic positions was 0.7. This allowed us to identify optimal fraction parameter when dealing with multi-allelic SNPs within MultiVCFAnalyzer, to boost the the number of semi-confident SNP positions (by including majority-call alleles in phylogenetically useful positions, under the assumptions majority calls derive from the target species of interest).
Figure R30 | Distributions of majority allele (i.e. >50%) frequency of multi-allelic SNPs for each genus, across all production dataset calculus mappings to single genomes of representative core microbial taxa. All mappings but Actinomyces (reference: Actinomyces dentalis DSM 19115) show that the most common highest frequency multi-allelic SNP bin is 70%. Calculated by selecting for each single-genome mapping the highest frequency multi-allelic SNP bin, from the 'SNP Table' of MultiVCFAnalyzer with a 'homozygous' threshold of 0.9, and 'heterozygous' threshold of 0.1 and minimum coverage threshold of 2.
Aggregation of this threshold parameter observation across all mappings can be
seen in 04-analysis/deep/competitive_mapping.backup/multiallelic_snps/.
For final variant calling for phylogenetic analysis, we re-ran MultiVCFAnalyzer to create our final SNP Alignments based on the single genome reference mappings.
We ran the same MultiVCFAnalyzer command as above, but with the slightly relaxed homozygous fraction parameter and turning off reporting of heterozygous positions by setting the heterozygous parameter to the same as the homozygous. Therefore we retained only 'confident' (not UPAC uncertainty) nucleotides in the alignments.
java -Xmx32g -jar MultiVCFanalyzer_0-87.jar \
NA \
"$FASTA" \
NA \
04-analysis/deep/multivcfanalyzer/superreference_mapping/output/superreference_mapping_2X_0.7_0.7/"$SPECIES" \
T \
30 \
2 \
0.7 \
0.7 \
NA \
<VCF_1> \
<VCF_2> \
<VCF_3>The final snpAlignment and snpStatistics files can be seen in 04-analysis/deep/multivcfanalyzer/initial_single_genome/output/initial_single_genome_2X_0.7_0.7/
⚠️ The remaining MultiVCFAnalyzer results are not provided here due to large size.
For the phylogenies themselves, I wrote a custom R script that allows for generating a few summary statistics for the alignment, filtering based on number of positions of an entry in the SNP alignment, and then pairwise-deletion neighbour-joining phylogenies. See main publication for justification behind the pairwise-deletion and NJ trees.
To ensure a enough positions are present for distance calculations between
samples we required a minimum of 1000 called positions for each sample, the
filtering of which is included in 02-scripts.backup/042-generate_NJ_tree.R.
e.g.
## Initial Single Genomes
for i in 04-analysis/deep/multivcfanalyzer/initial_single_genome/output/initial_single_genome_2X_0.7_0.7/*/snpAlignment.fasta.gz; do
echo "$i"
Rscript 02-scripts.backup/042-generate_NJ_tree.R "$i" 1000 JC69 100 none
echo ""
done
# 'cause Howlers are weird with P. gingivalis
Rscript 02-scripts.backup/042-generate_NJ_tree.R 04-analysis/deep/multivcfanalyzer/initial_single_genome/output/initial_single_genome_2X_0.7_0.7/Porphyromonas_gingivalis_ATCC_33277/snpAlignment.fasta.gz 2000 JC69 100 OME003We had to re-run Porphyromonas with a higher minimum position threshold, and excluded a sample. The higher position here was due to some samples having insufficient position overlap to calculate a genetic distance, and inclusion OME003 caused bootstrapping to fail as it had an unusually higher number of SNPs which ended up violating the equal base frequencies of the JC69 model.
The final Newick files and filtering statistics can be seen in
04-analysis/deep/multivcfanalyzer/initial_single_genome/output/initial_single_genome_2X_0.7_0.7/
Visualisation of the phylogenies were carried out with the R notebook
02-scripts.backup/026-Tree_visualisation_20190611.Rmd and PDF files of each
phylogeny for each of the taxa under 04-analysis/deep/phylogenies/plots and
combined below.
We selected the 8 best supported phylogenies at the deepest nodes of the tree, i.e. where the nodes representing the divergence of the host-genera had bootstrap values >=70%.
Figure R31 | Neighbour joining trees of eight well-supported calculus core taxa trees from single representative mappings of the production dataset. Trees generally show microbial strains clustering of individuals to those of the same host genus, and pre-14k BP European individuals consistently display a distinct clade from post-14k BP European individuals. Representative genomes were selected based on abundance and prevalence across all individuals in production dataset. SNPs were called using MultiVCFAnalyzer with a minimum coverage threshold of 2, and the majority allele threshold of 0.7. Alignments with <1000 called SNPs were removed. Genetic distance calculated using the ape R package, with the Jukes-Cantor 69 model and pairwise deletion strategy for missing data. Bootstraps are out of 100 bootstraps. Trees were selected as 'well-supported' if nodes resulting in expected host genus bifurcations equalled or exceeded 70%. Note that titles refer to the representative genome of the selected species used for the reference genome, and the alignments are mixtures of strains/species as indicated by high levels of multi-allelic sites. Grey boxes indicate European 'pre-14k BP' of the Red Lady of El Mirón and Neanderthals.
Comparison of the median fold depth of all mappings of a host genus, and
the pairwise number of overlapping bases between each sample can be seen in
02-scripts.backup/099-Phylogenies_SharedOverlappingSNPS.Rmd
You may need to
gunzipthe.nwkfiles before loading
Figure R32 | Relationship between number of positions shared, and fold depth coverage between pairwise combinations of individuals, from the production dataset mapped to representative core taxa. Higher coverage taxa generally display greater numbers of shared positions. Shared number of bases was calculated from the MultiVCFAnalyzer 'SNP alignment' FASTA file, with alignments containing less than 1000 bases removed. Order of microbial taxa and fill colour based on the genus median of average fold coverages average across all mappings in that genus as reported by EAGER. Boxplots present 25%, 50%, 75% of data, Dots represent outliers as calculated by the geom_histogram() function of ggplot. X axis is log scaled.
Overall we saw a general concordance whereby strains from the same host genera clustered together, but the relationships between these clades did not match the phylogenetic relationships between the hosts. See main publication for further discussion on the interpretation of the trees topologies.
In the trees generated above, we also observed that single deep sequenced pre-14ky BP individual (Red Lady/EMN001) always clustered with Neanderthals, whereas post-14ky BP individuals mostly fell with modern day humans.
One possible cause of this clustering could be that the two clades could represent different species (due to sub-optimal reference selection), and therefore the clustering of the Red Lady of El Mirón (EMN001) with the Neanderthals is because they share the same regions of the genome, which are not present in the other clade (leading to more similar distance calculations). Alternatively, the Neanderthals and EMN001 may have very small regions of the genome covered (given their age) and the distance calculated is just highly conserved regions with low diversity.
To check this, we built a distribution of the numbers of positions present in both of of all pair-wise combinations of humans individuals. Then we can calculated the median number of overlapping SNPs of EMN001 with each Neanderthals, and the same for each human individual - and see if the EMN001/Neanderthal median fell outside the range of the EMN001/Human and Human/Human combinations.
This is implemented in 'Version three' within the R notebook
02-scripts.backup/044-SNPAlignment_SharedData_Analysis_20190915.Rmd.
Figure R33 | Comparison of number shared positions of EMN001 and Neanderthals, compared to humans used to generate production dataset phylogenies. EMN001 and Neanderthal pairwise combinations do not show having shared number of positions falling outside the range of all human to human pairwise combinations. Histogram represents a count of all pairwise human combinations that have a given number of shared positions. All individuals with less than 1000 positions in each SNP alignment have been removed. Orange solid line represents median number of positions of EMN001 shared with each human, and red dashed line represents median shared between EMN001 and each Neanderthal individual.
The output files for can be seen in 04-analysis/deep/phylogenies/ with files
starting with SharedSNP*.
Overall we observed Neanderthals did not have any more or less SNP positions than other human combinations, suggesting clustering is not due to conserved or reconstruction of the wrong species genome used in the SNP alignment.
We also wanted to see if this clustering of the pre-14ky individual with Neanderthals held when adding additional European individuals, which was not available in the deep sequencing dataset.
For this, we attempted building the same phylogenies but with the screening counterparts of each sample (i.e. with damage, and lower coverage), with a few extra individuals i.e. PLV001 and RIG001 for pre-14ky BP, and OFN001 for post-14ky BP. While even though this would result in even lower resolution phylogenies compared the already simplified neighbour-joining trees, corroboration of topology (even if with lower bootstrap) could support a true biological signal regarding the observed pattern.
We set up EAGER the same way as above (retaining the stricter alignment parameters to try and reduce the effect of damage).
Once completed, we checked the coverage statistics of each EAGER run as in
02-scripts.backup/056-Phylogenies_Screening_EMNCheck_EAGERResults.Rmd.
The individual ReportTables can be seen in
04-analysis/screening/EMN_Neanderthal_phylogeny_check/multivcfanayzer/output.
Although coverage was low overall, we tried to build phylogenies anyway.
⚠️ The EAGER mapping results files are not provided here due to their large size other than the ReportTable files.
We again ran MultiVCFAnalyzer with the same parameters above, but to a new directory.
java -Xmx32g -jar MultiVCFanalyzer_0-87.jar \
NA \
"$FASTA" \
NA \
04-analysis/screening/EMN_Neanderthal_phylogeny_check/multivcfanalyzer/output/"$SPECIES" \
T \
30 \
2 \
0.7 \
0.7 \
NA \
<VCF_1> \
<VCF_2> \
<VCF_3>Then same as above, we attempted to recreate the same 8 better-supported deep sequenced data phylogenies with:
for i in 04-analysis/screening/EMN_Neanderthal_phylogeny_check/multivcfanalyzer/output/*/snpAlignment.fasta.gz; do
echo "$i"
Rscript 02-scripts.backup/042-generate_NJ_tree.R "$i" 1000 JC69 100 none
echo ""
doneThe results can be seen in 04-analysis/screening/EMN_Neanderthal_phylogeny_check/multivcfanayzer/output
⚠️ Only a subset of MultiVCFAnalyzer files are uploaded here, due to large size
With these we can load into the R markdown document
058-Tree_visualisation_20191025_screening.Rmd to see the trees. The PDF
figures can be seen under 04-analysis/screening/EMN_Neanderthal_phylogeny_check/phylogenies
You may need to
gunzipthe.nwkfiles before loading
Figure R34 | Replication of production dataset phylogenies with low-coverage and damage-containing screening dataset with additional European individuals. The observed pattern of pre-14k BP Europeans and post-14k BP humans clustering separately in the production dataset phylogenies is replicated when including additional pre- and post-14k BP individuals when using screening dataset equivalents. Representative genomes were selected based on abundance and prevalence across all individuals in production dataset. SNPs were called using MultiVCFAnalyzer with a minimum coverage threshold of 2, and the majority allele threshold of 0.7. Alignments with <1000 called SNPs were removed. Genetic distance calculated using the ape R package, with the Jukes-Cantor 69 model and pairwise deletion strategy for missing data. Bootstraps are out of 100 bootstraps. Grey boxes indicate European 'pre-14k BP' of the Red Lady of El Mirón and Neanderthals
In general we saw, albeit at lower resolution, similar patterns where the pre-14ky individuals still fell with the Neanderthal individuals, while the post-14k individuals fell with other ancient and/or modern humans.
In addition to taxonomic relationships in the calculus microbiomes of each host genus we wanted to explore overall functional differences, and also more to see if we could traces changes virulence factors of what is typically considered 'pathogenic' red complex taxa (but is increasingly being shown to be either opportunistic commensals or having many commensal relatives) over the evolution of Hominids.
Given that we identified in the production dataset significant numbers of reads mapping to reference genomes of well-studied 'red complex' species, classically considered pathogens, across most host genera, we were interested to see if we would observe any change in the pathogenicity based on gain/loss of identified virulence factors in human strains.
For this we used the production mapping datasets to the references of Tannerella forsythia and Porphyromonas gingivalis.
We took the deduplicated bedfiles and run bedtools coverage on them, using
a collection of virulence factors described in the literature. This list
including NCBI gene locus tags can be seen in 06-additional_data_files under
Data R35.
bedtools coverage -a <REFERENCE>.gff -b <BAM> > <OUT FILE>.tsv
bedtools coverage -a <REFERENCE>.gff -b <BAM> > <OUT FILE>.tsv -meanFor the actual filtering of individuals with overall genomic low coverage (i.e.
retaining individuals that had sufficient coverage that we could say with
reasonable confidence of a true presence- or absence - Figure R35) and the
actual identification of presence/absence of these genes, we loaded the
resulting TSV files into R with the notebook
02-scripts.backup/054-virulence_investigation.Rmd.
Figure R35 | Distribution of (annotated) gene counts passing a breadth threshold of 70%, as calculated from the mappings of the production dataset to two 'red complex' bacteria reference genomes. A clear cut off of ~500 genes can be seen, reflecting the separation between low-coverage and higher coverage genomes.
Figure R36 | Heatmap of ratios (fill) of virulence gene depth coverage and gene completeness (size), in mappings of the production dataset to two 'red complex' bacteria reference genomes. Virulence factors associated with oral disease are found prevalent across all host genera.
For Tannerella forsythia we saw that most virulence factors were present within most individuals of the deep sequenced dataset, whereas there was more variation in in P. gingivalis, with the Mfa family of fimbrae genes seemingly missing in Alouatta and Pan.
In the hierarchical clustering (above), we observed the relative abundance and prevalence of streptococci varied between host genus. We therefore wished to explore this further - given the interest in human evolutionary history regarding the role of amylase copy number variation (see main publication for an overview), and certain groups of streptococci displaying amylase-binding activity.
Firstly, we looked at the distribution of different types of streptococci groups
within each of our host genera. We generated a 'consensus' table of streptococci
groups and their reported amylase-binding protein activity (as reported in the
literature), described here in 06-additional_data_files under Data R33 or
under 00-documentation.backup/25-streptococcus_cladegroup_amylasegroup_database.tsv.
Using the MALT species level OTU tables from the screening dataset, we
visualised as stacked bar plots the proportion of Streptococcus alignments
deriving from each group (Figure R64). We can also applied the same concept to the production
dataset - but using the Streptococcus super-reference as the reference
database (Figure R37), as in the notebook
02-scripts.backup/033-streptoccocus_investigation_v2_20190826.Rmd.
Figure R64 | Distribution of screening dataset assignments to each Streptococcus group when mapping to a the NCBI nt database with MALT, normalised by the total number of MALT assignments to all Streptococci Stacked bar plot of proportion of alignments to different Streptococcus groups show differences between host genera. Proportion is calculated over all streptococci reads as identified using MALT to the NCBI nt database (at species level after low abundance removal of taxa with ≤ 0.04% of all alignments). One Gorilla was removed, as it had no signal of Streptococcus. Individuals ordered by population/time period, except Homo individuals resembling Pan. Amylase-binding activity has been observed among members of the Sanguinis, Mitis and Salivarius groups.
Figure R37 | Distribution of production dataset reads to each Streptococcus group when mapping to a Streptococcus super-reference, normalised by the total number of Streptococcus aligned reads. As with the screening dataset, Homo are dominated by streptococci groups displaying amylase binding protein activity (sangiuinis, mitis and salivarius). See section 6. Microbial phylogenetics for details on super-reference construction and mapping. Streptococcus groups are ordered by amylase activity as reported by Haase 2017 BMC Microbiol - in which members within the sanguinis, mitis and salivarius groups showed amylase activity.
In both the screening and deep sequencing datasets, we observed that Homo had greater fractions of alignments to streptococci that have been empirically shown to display amylase-binding activity compared to other host genera (although also with a greater level of overall streptococci reads aligning to untested taxa in the non-Homo hosts)
Given the different distributions of the Streptococcus content of each host genus, and the observation that Humans tend to have more prominent signals of amylase-binding activity-positive species, we also looked at whether amylase- binding genes could actually be detected within each host genus.
We decided to use the production dataset for this, as it would provide higher confidence that a gene is present, given the higher whole-depth depth coverages in this dataset. Given that sections of amylase-binding protein B (abpB) sequence are present in other genes (and variants in other species), we also wanted to find all possible 'amylase-binding-protein gene'-like sequences in the super-reference. So as to maximise our sensitivity in finding reads putatively deriving from the actual amylase binding protein gene.
For this we recovered all amylase-like reads from our Streptococcus super-reference with the tool panX, using the Genbank files for each reference.
## Run panX analysis
pan-genome-analysis/panX.py \
-fn <GENBANK_FILE_DIR> \
-sl Streptococcus_Superreference \
-t 32Plugging the output into the panX visualisation companion tool (see the panX
documentation, we
searched for the abpA and abpB genes as annotated in the well-annotated
Streptococcus gordonii genome, and downloaded the corresponding FASTA
alignments of similar sequences from the sequence alignment table. The
alignments can be seen under
04-analysis/screening/streptococcus_investigation.backup/panX/abpA_abpB_cluster_alignments.
We then performed a filtering procedure to the files due to
the inclusion of highly diverged sequences (as we ran panX on default settings),
as described in
02-scripts.backup/048-panX_streptococcus_amylasebindingproteincluster_detection.Rmd.
The final list of annotations can be seen in 06-additional_data_files under
Data R34.
We then used the R script 02-scripts.backup/049-Streptoccocus_superreference_amylasebinding_coordinate_reconstruction.R
to recover the coordinates of these sequences from the super-reference FASTA,
which was exported as a BED file.
⚠️ The super-reference FASTA files are not included here to large size.
For each sample's mapping to the Streptococcus super-reference, we then ran bedtools to recover statistics on the coverage of each annotated gene entry.
## Extract reads from abpA and abpB-like regions
bedtools intersect \
-c \
-a <ABPA_OR_ABPB_BEDFILE> \
-b <SUPER-REFERENCE_BAM>The resulting files were then loaded into to assess the ratio of all
Streptococcus reads to amylase binding protein-like reads as in
02-scripts.backup/051-streptococcus_superreference_to_amylase_comparison.Rmd.
The distributions can be seen in Figure R65.
We observed that calculated ratios were much higher in Homo (with Neanderthals falling within the range of humans for abpB) than other primates. See the main publication for the implications of this. This was also statistically confirmed with Mann-Whitney U tests (see R notebook), which were also compared to a distribution of p-values of 100 random shuffle of group assignments (see Figure R63 below).
Figure R65 | Ratios of reads aligning to amylase-binding-protein annotated sequences versus a ‘super-reference’ of 166 Streptococcus assemblies show higher values in Homo than non-human primates. Distributions based on a deep-sequenced subset of samples and four present-day humans. Note the ratio on the Y axes of abpA and abpB are scaled differently due to the greater number of abpB-like sequences in the super-reference and therefore more reads are likely to be retrieved.
Figure R63 | Comparison of Mann-Whitney-U p-value of Homo and non-Homo abp to Streptococcus super-reference reads against a distribution of randomised sample group assignments Red line indicates result of test with 'true' group assignments. Dotted and dashed lines represent alphas of 0.05 and 0.01 respectively. Violin distribution represents p-values of 100 shuffles of group assignments of samples (i.e. whether a sample is from Homo and non-Hpmo groups), with 25%, 50%, and 75% quantiles. The true results fall well outside distribution of p-values from shuffled assignments, which in principle reflects 'random noise'.
Note: the figure ID is out of order due to missing upload during initial writing of this walk-through.
Given this pattern, we were interested if we could infer any more information about the evolutionary history of these genes.
We therefore identified reads in the screening dataset originating from specifically the abpA and abpB genes by independently using BLAST and mapping against a selected set of reference sequences of abpA and abpB.
All reference abpA sequences in fasta format were built into a blast database:
makeblastdb -in <abp_gene>.fasta -dbtype nucl -title <abp_gene> -out <abp_gene>and then all screening samples were searched against both databases using blast command line tools:
## ran once with <abp_database> for abpA and again with <abp_database> for abpB
gzip -dcf <SAMPLENAME>.fastq.gz | blastn -db <abp_database> -query - -out $(basename <SAMPLENAME> .fasta.gz).out -num_descriptions 20 -num_alignments 20 -num_threads 12All reads that hit any reference sequence were extracted from a blast format
output file to a list using the script ncbiblastparser.pl downloaded
from https://github.com/mel-astar/mel-ngs/blob/master/utils/ncbiblastparser.pl
ncbiblastparser.pl <(gzip -dcf <SAMPLENAME>.out) 1 "$(echo <SAMPLENAME>)".parsed
grep -v 'No hits found' "$(echo <SAMPLENAME>)".parsed > "$(echo <SAMPLENAME>)".hitsThe read ID of all sequences with a hit in the blast output were copied to a list
awk '{print $1}' <SAMPLENAME>.hits > <SAMPLENAME>.listThis list was used to extract the reads from the original fastq files using seqtk into new files to be mapped against apbA and abpB references sequences:
zcat <SAMPLENAME>.fastq.gz | seqkit grep -f <SAMPLENAME>.list > <SAMPLENAME>.fastqThe individual abpA and abpB sequences used for the BLAST database were then indexed with bwa for mapping:
for f in *.fasta; do
bwa index -p `basename $f .fasta` $f
doneEach BLAST-hit read sample file was mapped against each abpA and abpB reference sequence:
bwa aln -n 0.01 -l 32 <reference_abp_sequence> <SAMPLENAME>.fastq > <SAMPLENAME>.abp_reference_seqence.sai
bwa samse <reference_abp_sequence> <SAMPLENAME>.abp_reference_seqence.sai <SAMPLENAME> > <SAMPLENAME>.abp_reference_seqence.samFinally files were converted to bam format, mapped reads selected out, duplicate reads removed from the mapped read only file, and the duplicate-removed mapped-only read file was indexed with the following procedure:
# convert sam to bam
for f in *.sam; do
samtools view -bS $f | samtools sort - $(basename $f .sam).bam
done
# select only mapped reads
for f in *.bam; do
samtools view -b -F4 $f > $(basename $f .bam).mapped.bam
done
# remove duplicates from mapped reads
for f in *mapped.bam; do
samtools rmdup -s $f $(basename $f .bam)_rmdup.bam 2>&1 >/dev/null | cut -d' ' -f 6 > $(basename $f .bam)_dup.txt # collects read IDs of the duplicate reads
samtools index $(basename $f .bam)_rmdup.bam
done
# index the mapped, dupliate-removed bam files
for f in *rmdup.bam; do
samtools index $f $(basename $f .bam).bai
doneWe performed additional mapping using looser parameters to see if we picked up additional reads mapping to either apbA or abpB that were missed with our standard mapping parameters. This way we were able to make sure we were not missing variation in the genes due to evolutionary changes, particularly in the non-Homo groups. We counted the number of reads that mapped to each reference sequence in each sample, and compared it to the number that mapped using standard paremeters. We found that there were no more than 2 additional reads mapped per reference sequence per sample, and in no cases did this bring coverage to >40%.
bwa aln -n 5 -l 1000 <reference_abp_sequence> <SAMPLENAME>.fastq > <SAMPLENAME>.abp_reference_seqence.sai
bwa samse <reference_abp_sequence> <SAMPLENAME>.abp_reference_seqence.sai <SAMPLENAME> > <SAMPLENAME>.abp_reference_seqence.sam
# convert sam to bam
for f in *.sam; do
samtools view -bS $f | samtools sort - $(basename $f .sam).bam
done
# select only mapped reads
for f in *.bam; do
samtools view -b -F4 $f > $(basename $f .bam).mapped.bam
done
# remove duplicates from mapped reads
for f in *mapped.bam; do
samtools rmdup -s $f $(basename $f .bam)_rmdup.bam 2>&1 >/dev/null | cut -d' ' -f 6 > $(basename $f .bam)_dup.txt # collects read IDs of the duplicate reads
samtools index $(basename $f .bam)_rmdup.bam
done
# index the mapped, dupliate-removed bam files
for f in *rmdup.bam; do
samtools index $f $(basename $f .bam).bai
done
# count the # of mapped, non-duplicate reads
for f in *rmdup.bam; do
samtools view $f | wc -l
doneAll alignments from mapping with the standard parameters (-n 0.01 -l 32) were visually inspected with IGV, and consensus sequences from mapping against the Streptococcus gordonii str. Challis (NC_009785) reference abpA and abpB sequences were exported from IGV if they covered at least 40% of the reference at least 1X. To do this, on the alignment track we right-clicked to bring up a menu and select 'Copy consensus sequence'. All consensus sequences were then pasted into a text file in fasta format that included the NC_009785 reference sequence, and this was used as an alignment file. The consensus sequence fasta file was uploaded to Geneious v 8.0.5 and exported in a nexus file format.
The consensus sequence nexus file was uploaded into BEAUTi, for Bayesian skyline analysis with BEAST2 v 2.4.7., and dates were added, either as estimated from archaeological context (see main publication and extended data ), or from known collection dates of modern data. All parameters were left at default with the GTR substitution model, a strict clock, and coalescent Bayesian skyline model. The MCMC chain length was 800,000,000 with sampling every 80,000 states.
While the chain converged, inspection of the tree structure in DensiTree showed no structure for abpA, and we therefore did not proceed with BEAST analysis to attempt to date expansion of abpA. However, we generated a skyline plot from the abpB data, which is shown in Figure R38.
A list of accession numbers of abpB sequences collected for bayesian skyline
analysis can be seen in 06-additional_data_files under Data R36.
Figure R38 | Bayesian skyline plot of population expansion of the amylase-binding protein B gene (abpB). The population shows expansion at ~7000 years before the present, with a decline at around 500 years before the present. However, the uneven distribution of samples across time make the results unreliable, and we do not draw any conclusions from these results.
Due to low and uneven sample sizes, we were unable to draw confident conclusions from these results.
In addition to the taxonomic profile, the functional profile of dental calculus microbiota may provide insight into host evolutionary patterns. The functional profile of a metagenomic sample is determined by the gene content of species that are present. The program HMP Unified Metabolic Analysis Network 2 (HUMAnN2, Franzosa, et al. 2018) was developed to generate a metabolic functional profile from a metagenome sample. HUMAnN2 provides both species-specific gene assignments and general gene assignments, when the species of origin cannot be determined, as well as grouping the genes into the metabolic pathways they contribute to and providing a metabolic pathway profile. We assessed both the metabolic pathway profile and the gene content profile of our samples generated by HUMAnN2, as detailed below.
In preparation for HUMAnN2, we ran MetaPhlAn2, running on the whole screening dataset.
INDIR=04-analysis/screening/metaphlan2/input
OUTDIR=04-analysis/screening/metaphlan2/output
for LIBFILE in "$INDIR"/*/*.fq.gz; do
LIBNAME=$(echo "$LIBFILE" | rev | cut -d/ -f2 | rev)
if [ -d "$OUTDIR"/"$LIBNAME" ]; then
printf "\n $LIBNAME already Processed \n\n"
else
mkdir "$OUTDIR"/"$LIBNAME"
metaphlan2.py $LIBFILE \
-o $OUTDIR/$LIBNAME/$LIBNAME.mp2profile.tsv \
--input_type fastq \
--nproc 2 \
-t rel_ab
fi
done
## if re-running with different parameters, here changing fastq to bowtie2out
INDIR=04-analysis/screening/metaphlan2/input
OUTDIR=04-analysis/screening/metaphlan2/output
for LIBFILE in "$INDIR"/*/*bowtie2out.txt; do
LIBNAME=$(echo "$LIBFILE" | rev | cut -d/ -f2 | rev)
if [ -d "$OUTDIR"/"$LIBNAME" ]; then
printf "\n $LIBNAME already Processed \n\n"
else
mkdir "$OUTDIR"/"$LIBNAME"
metaphlan2.py $LIBFILE \
-o $OUTDIR/$LIBNAME/$LIBNAME.mp2profile.tsv \
--input_type bowtie2out \
--nproc 2 \
-t rel_ab
fi
done
## Re-running to get estimated read counts with rel_ab to rel_ab_w_read_stats
INDIR=04-analysis/screening/metaphlan2/input
OUTDIR=04-analysis/screening/metaphlan2/output_readcounts
for LIBFILE in "$INDIR"/*/*bowtie2out.txt; do
LIBNAME=$(echo "$LIBFILE" | rev | cut -d/ -f2 | rev)
if [ -d "$OUTDIR"/"$LIBNAME" ]; then
printf "\n $LIBNAME already Processed \n\n"
else
mkdir "$OUTDIR"/"$LIBNAME"
metaphlan2.py $LIBFILE \
-o $OUTDIR/$LIBNAME/$LIBNAME.mp2profile_readstats.tsv \
--input_type bowtie2out \
--nproc 2 \
-t rel_ab_w_read_stats
fi
done
## Re-running to get actual rad counts with rel_ab to rel_ab_w_read_stats
INDIR=04-analysis/screening/metaphlan2/input
OUTDIR=04-analysis/screening/metaphlan2/output_readmappedcounts
for LIBFILE in "$INDIR"/*/*bowtie2out.txt; do
LIBNAME=$(echo "$LIBFILE" | rev | cut -d/ -f2 | rev)
if [ -d "$OUTDIR"/"$LIBNAME" ]; then
printf "\n $LIBNAME already Processed \n\n"
else
mkdir "$OUTDIR"/"$LIBNAME"
metaphlan2.py $LIBFILE \
-o $OUTDIR/$LIBNAME/$LIBNAME.mp2profile_readsmappedstats.tsv \
--input_type bowtie2out \
--nproc 2 \
-t reads_map
fi
doneTo merge all of the MetaPhlAn2 percentage profiles of all the libraries we then did the following.
cd 04-analysis/screening/metaphlan2/output
## Note, remove metaphlan2.py script in the variable here as using util scripts
METAPHLAN2=biobakery-metaphlan2-7898bf1/
INDIR=04-analysis/screening/metaphlan2/input
OUTDIR=04-analysis/screening/metaphlan2/output
"$METAPHLAN2"/utils/merge_metaphlan_tables.py "$OUTDIR"/*/*mp2profile.tsv > "$OUTDIR"/mp2_merged_abundance_table_all_"$(date +%Y%m%d)".txtFor merging of the estimated read count files, we ran the
016-metaphlan2_readcount_table_generator.Rmd notebook.
For merging of the 'mapped reads method', the output actually gave both the name of the read and a given taxonomic ID. To merge we thus ran:
for i in */*; do
echo $(echo "$i" | cut -d "/" -f 1) $(zcat "$i" | wc -l) ;
done > mp2_merged_readsmapped_table_all_"$(date +%Y%m%d)".txt
Note that all of those files needed to be -1 because the count included a header.
⚠️ Individual MetaPhlAn2 files are not provided here due to redundancy with combined file(s)
The summarised output MetaPhlAn2 files can be seen in 06-additional_data_files
under Data R15.
Finally, some read statistics by applying the same 0.01% threshold used in MALT
were gained via the 099-MetaPhlan2_Summary_statistics.R script. These were
then manually added to the main analysis metadata file (Data R04 in
06-additional_data_files).
Figure R39 | Percentage of non-human reads mapping to the MetaPhlAn2 database. Colours correspond to calculus host genus. Blue: Alouatta; Purple: Gorilla; Green: Pan; Orange: Homo; Grey: non-calculus.
Figure R40 | Distributions of the number of genera and species identified by MetaPhlAn2 across each study group. Colours correspond to calculus host genus. Blue: Alouatta; Purple: Gorilla; Green: Pan; Orange: Homo; Grey: non-calculus.
The numbers of reads with taxonomic assignment were across all taxa, as expected from a) the marker-gene profiling approach used by MetaPhlAn2 and b) ancient samples with large amounts of 'unknown' environmental contamination. Interestingly, the level of reads identified in Plaque samples was much more high and consistent than all other groups.
Once we had the MetaPhlAn2 profiles, we ran run HUMAnN2 with the following command.
⚠️ The following was adapted from a SLURM array script and should be adjusted accordingly to run on each sample
humann2 \
--input <INPUT_SAMPLE>.fq.gz \
--output 04-analysis/screening/humann2/output/"$SAMPLENAME"/ \
--taxonomic-profile <INPUT_MP2_PROFILE>.tsv \
--threads 8 \
--memory-use maximum
cd 04-analysis/screening/humann2/output
rm -r */*_temp/which reduced our footprint from 4.9 TERABYTES(!?!?!) down to 3.9 gigabytes. This is mostly due to the uncompressed SAM files.
Next we needed to normalise the data of each file. We only needed to do this on the 'genefamilies' and 'pathabundance' data, as you don't have to do this for the pathwaycoverage
cd 04-analysis/screening/humann2/output
for SAMPLE in */*_genefamilies.tsv; do
humann2_renorm_table --input $SAMPLE --output ${SAMPLE%.tsv}_cpm.tsv --units cpm --update-snames
done
for SAMPLE in */*_pathabundance.tsv; do
humann2_renorm_table --input $SAMPLE --output ${SAMPLE%.tsv}_cpm.tsv --units cpm --update-snames
done
rm */*_genefamilies.tsv
rm */*_pathabundance.tsv
# -s to search subdirectories of current directory to look for the files
humann2_join_tables --input . --output humann2_genefamilies.tsv --file_name genefamilies_cpm -s
humann2_join_tables --input . --output humann2_pathcoverage.tsv --file_name pathcoverage -s
humann2_join_tables --input . --output humann2_pathabundance.tsv --file_name pathabundance_cpm -s
We also wanted to put the output in KEGG format. To do this we firstly downloaded the re-grouping database
humann2_databases --download utility_mapping full 01-data/databases/humann2
And then regrouped our table(s) accordingly
humann2_regroup_table -i humann2_pathabundance.tsv -g uniref90_ko -o humann2_pathabundance_ko.tsv
humann2_regroup_table -i humann2_genefamilies.tsv -g uniref90_ko -o humann2_genefamilies_ko.tsv
⚠️ HUMANn2 output files are not provided here due to large size
All in-depth analysis and figure generation for HUMAnN2 data can be found in the
R markdown document here
02-scripts.backup/144-imv-oral_evolution_humann2_fxn_cleaned.Rmd. However,
a brief overview with figures can be seen here.
We used pathway abundance data to perform PCAs, which were plotted to visualize the relationships between samples and controls, as well as within and between samples. The samples that plotted with controls, except plaque, were removed as outliers with poor preservation (File R37).
 Figure R41 | Principal components analysis of pathway abundances identified by HUMAnN2. PCA of pathway abundances with a all samples and controls, b outlier samples removed, c only plaque and calculus samples, d only calculus samples. Outliers were determined for pathway abundances based on plotting with the controls samples (not plaque) in panel A.
Figure R41 | Principal components analysis of pathway abundances identified by HUMAnN2. PCA of pathway abundances with a all samples and controls, b outlier samples removed, c only plaque and calculus samples, d only calculus samples. Outliers were determined for pathway abundances based on plotting with the controls samples (not plaque) in panel A.
The differences in the number of assignments to UniRef90 categories, KEGG orthologs, and KEGG carbohydrate orthologs between sample groups were explored.
 Figure R42 | HUMAnN2 read assignment statistics. All graphs have outlier samples removed. a Percent of reads assigned to a UniRef90 protein, outlier samples removed. b Proportion of UniRef90 assignments that grouped to KEGG orthologs. c The total number of KEGG orthologs in any of the 15 KEGG Carbohydrate pathways in each sample. d Abundance of KEGG orthologs in any of the 15 KEGG Carbohydrate pathways in each sample. * P < 0.05, ** P < 0.01, *** P<0.001.
Figure R42 | HUMAnN2 read assignment statistics. All graphs have outlier samples removed. a Percent of reads assigned to a UniRef90 protein, outlier samples removed. b Proportion of UniRef90 assignments that grouped to KEGG orthologs. c The total number of KEGG orthologs in any of the 15 KEGG Carbohydrate pathways in each sample. d Abundance of KEGG orthologs in any of the 15 KEGG Carbohydrate pathways in each sample. * P < 0.05, ** P < 0.01, *** P<0.001.
We used KEGG ortholog abundance data to perform PCAs, which were plotted to visualize the relationships between samples and controls, as well as within and between samples. The samples that plotted with controls, except plaque, were removed as outliers with poor preservation (File R37).
 Figure R43 | Principal components analysis of KEGG orthologs separates host genera. PCA of pathway abundances with a all samples and controls, b outlier samples removed, c only plaque and calculus samples, d only calculus samples. Outliers were determined for pathway abundances based on plotting with the controls samples (not plaque) in panel A.
Figure R43 | Principal components analysis of KEGG orthologs separates host genera. PCA of pathway abundances with a all samples and controls, b outlier samples removed, c only plaque and calculus samples, d only calculus samples. Outliers were determined for pathway abundances based on plotting with the controls samples (not plaque) in panel A.
Biplots were used to visualize the KEGG orthologs with strongest loadings in PC1 and PC2, to understand what drives separation of sample groups. The table with these loadings is File R38.
 Figure R44 | PCA biplots with the KEGG orthologs (Figure R39/SM3D above) with the top 10 strongest positive and negative loadings on PC1 and PC2. a Top loadings of PC1 with all Homo samples. b Top loadings of PC1 without present-day Homo samples. c Top loadings of PC2 with all Homo samples. d Top loadings of PC2 without present-day Homo samples. Note in panels b and d (without present-day Homo samples) that the y-axis has been reversed to maintain orientation with the other PCAs.
Figure R44 | PCA biplots with the KEGG orthologs (Figure R39/SM3D above) with the top 10 strongest positive and negative loadings on PC1 and PC2. a Top loadings of PC1 with all Homo samples. b Top loadings of PC1 without present-day Homo samples. c Top loadings of PC2 with all Homo samples. d Top loadings of PC2 without present-day Homo samples. Note in panels b and d (without present-day Homo samples) that the y-axis has been reversed to maintain orientation with the other PCAs.
The abundance of each of the orthologs plotted in the biplots above were visualized in heat maps to visualize the difference between host groups, including modern Homo samples.
 Figure R45 | Heat maps showing the centered log ratio-transformed abundance of the top 10 proteins with strongest loadings from the PCA including present-day Homo. Host genus is indicated by the colored bars to the right, where blue is Alouatta, purple is Gorilla, green is Pan, and orange is Homo. Symbols in Homo indicate different groups, where triangle pointed up is Neanderthal, square is pre-agricultural human, circle is pre-antibiotic human, and triangle pointed down is modern day human. a PC1 positive top 10 KEGG orthologs with strongest loadings. b PC1 negative top 10 KEGG orthologs with strongest loadings. c PC2 positive top 10 KEGG orthologs with strongest loadings. d PC2 negative top 10 KEGG orthologs with strongest loadings.
Figure R45 | Heat maps showing the centered log ratio-transformed abundance of the top 10 proteins with strongest loadings from the PCA including present-day Homo. Host genus is indicated by the colored bars to the right, where blue is Alouatta, purple is Gorilla, green is Pan, and orange is Homo. Symbols in Homo indicate different groups, where triangle pointed up is Neanderthal, square is pre-agricultural human, circle is pre-antibiotic human, and triangle pointed down is modern day human. a PC1 positive top 10 KEGG orthologs with strongest loadings. b PC1 negative top 10 KEGG orthologs with strongest loadings. c PC2 positive top 10 KEGG orthologs with strongest loadings. d PC2 negative top 10 KEGG orthologs with strongest loadings.
The abundance of each of the orthologs plotted in the biplots above were visualized in heat maps to visualize the difference between host groups, excluding modern Homo samples.
 Figure R46 | Heat maps showing the centered log ratio-transformed abundance of the top 10 proteins with strongest loadings from the PCA excluding present-day Homo. Host genus is indicated by the colored bars to the right, where blue is Alouatta, purple is Gorilla, green is Pan, and orange is Homo. Symbols in Homo indicate different groups, where triangle pointed up is Neanderthal, square is pre-agricultural human, and circle is pre-antibiotic human. a PC1 positive top 10 KEGG orthologs with strongest loadings. b PC1 negative top 10 KEGG orthologs with strongest loadings. c PC2 positive top 10 KEGG orthologs with strongest loadings. d PC2 negative top 10 KEGG orthologs with strongest loadings.
Figure R46 | Heat maps showing the centered log ratio-transformed abundance of the top 10 proteins with strongest loadings from the PCA excluding present-day Homo. Host genus is indicated by the colored bars to the right, where blue is Alouatta, purple is Gorilla, green is Pan, and orange is Homo. Symbols in Homo indicate different groups, where triangle pointed up is Neanderthal, square is pre-agricultural human, and circle is pre-antibiotic human. a PC1 positive top 10 KEGG orthologs with strongest loadings. b PC1 negative top 10 KEGG orthologs with strongest loadings. c PC2 positive top 10 KEGG orthologs with strongest loadings. d PC2 negative top 10 KEGG orthologs with strongest loadings.
We looked at whether specific species contributed the orthologs with strongest loadings in the biplots above to look for host-specific species contributions to orthologs. First we looked at the orthologs in strongest positive loadings in PC1.
 Figure R47 | Microbial genus contributions to KOs with the strongest positive loadings in PC1. Bar graphs of the genera, summed from species, that contribute >12% to the KEGG orthologs with strongest positive loadings in PC1: a Including modern Homo samples, grouped by KEGG ortholog, b Including present-day Homo samples, grouped by host genus, c Excluding present-day Homo samples, grouped by KEGG ortholog, and d Excluding present-day Homo samples, grouped by host genus. All genera that individually contributed <12% are grouped together as g__Other.
Figure R47 | Microbial genus contributions to KOs with the strongest positive loadings in PC1. Bar graphs of the genera, summed from species, that contribute >12% to the KEGG orthologs with strongest positive loadings in PC1: a Including modern Homo samples, grouped by KEGG ortholog, b Including present-day Homo samples, grouped by host genus, c Excluding present-day Homo samples, grouped by KEGG ortholog, and d Excluding present-day Homo samples, grouped by host genus. All genera that individually contributed <12% are grouped together as g__Other.
We looked at whether specific species contributed the orthologs with strongest loadings in the biplots above to look for host-specific species contributions to orthologs. Then we looked at the orthologs in strongest negative loadings in PC1.
 Figure R48 | Microbial genus contributions to KOs with the strongest negative loadings in PC1. Bar graphs of the genera, summed from species, that contribute >12% to the KEGG orthologs with strongest negative loadings in PC1: a Including modern Homo samples, grouped by KEGG ortholog, b Including present-day Homo samples, grouped by host genus, c Excluding present-day Homo samples, grouped by KEGG ortholog, and d Excluding present-day Homo samples, grouped by host genus. All genera that individually contributed <12% are grouped together as g__Other.
Figure R48 | Microbial genus contributions to KOs with the strongest negative loadings in PC1. Bar graphs of the genera, summed from species, that contribute >12% to the KEGG orthologs with strongest negative loadings in PC1: a Including modern Homo samples, grouped by KEGG ortholog, b Including present-day Homo samples, grouped by host genus, c Excluding present-day Homo samples, grouped by KEGG ortholog, and d Excluding present-day Homo samples, grouped by host genus. All genera that individually contributed <12% are grouped together as g__Other.
We looked at whether specific species contributed the orthologs with strongest loadings in the biplots above to look for host-specific species contributions to orthologs. Then we looked at the proteins in strongest PC2 loadings characterizing non-human primates.
 Figure R49 | Microbial genus contributions to KOs with the strongest loadings in PC2 characterizing Pan/Gorilla/Alouatta. Bar graphs of the genera, summed from species, which contribute >12% to the KEGG orthologs with strongest positive loadings in PC2: a Including present-day Homo, grouped by KEGG ortholog, b Including present-day Homo, grouped by host genus, c and the strongest negative loadings in PC2 excluding present-day Homo, grouped by KEGG ortholog, and d Excluding present-day Homo, grouped by host genus. All genera that individually contributed <12% are grouped together as g__Other.
Figure R49 | Microbial genus contributions to KOs with the strongest loadings in PC2 characterizing Pan/Gorilla/Alouatta. Bar graphs of the genera, summed from species, which contribute >12% to the KEGG orthologs with strongest positive loadings in PC2: a Including present-day Homo, grouped by KEGG ortholog, b Including present-day Homo, grouped by host genus, c and the strongest negative loadings in PC2 excluding present-day Homo, grouped by KEGG ortholog, and d Excluding present-day Homo, grouped by host genus. All genera that individually contributed <12% are grouped together as g__Other.
We looked at whether specific species contributed the orthologs with strongest loadings in the biplots above to look for host-specific species contributions to orthologs. Last we looked at the proteins in strongest PC2 loadings characterizing Homo.
 Figure R50 | Microbial genus contributions to KOs with the strongest loadings in PC2 in the direction characterizing Homo. Bar graphs of the genera, summed from species, that contribute >12% to the KEGG orthologs with strongest negative loadings in PC2 a Including present-day Homo, grouped by KEGG ortholog, b Including present-day Homo, grouped by host genus, c Excluding present-day Homo, grouped by KEGG ortholog, and d Excluding present-day Homo, grouped by host genus. All genera that individually contributed <12% are grouped together as g__Other.
Figure R50 | Microbial genus contributions to KOs with the strongest loadings in PC2 in the direction characterizing Homo. Bar graphs of the genera, summed from species, that contribute >12% to the KEGG orthologs with strongest negative loadings in PC2 a Including present-day Homo, grouped by KEGG ortholog, b Including present-day Homo, grouped by host genus, c Excluding present-day Homo, grouped by KEGG ortholog, and d Excluding present-day Homo, grouped by host genus. All genera that individually contributed <12% are grouped together as g__Other.
Lastly, we looked at whether KEGG orthologs in specific metabolic pathways were as distinct between host groups as all KEGG orthologs combined, by performing and plotting PCAs with KEGG orthologs from only the Carbohydrate, Amino acid, and Lipid metabolic pathways independently.
 Figure R51 | PCAs using KEGG orthologs belonging to specific metabolic pathway categories. KEGG orthologs in pathways that process major biomolecules all separate samples by host genus in a pattern similar to that seen in taxonomy, metabolic pathways, and all KEGG orthologs. a All KEGG orthologs in the Carbohydrate metabolism pathways. b All KEGG orthologs in the Amino acid metabolism pathways. c All KEGG orthologs in the Lipid metabolism pathways.
Figure R51 | PCAs using KEGG orthologs belonging to specific metabolic pathway categories. KEGG orthologs in pathways that process major biomolecules all separate samples by host genus in a pattern similar to that seen in taxonomy, metabolic pathways, and all KEGG orthologs. a All KEGG orthologs in the Carbohydrate metabolism pathways. b All KEGG orthologs in the Amino acid metabolism pathways. c All KEGG orthologs in the Lipid metabolism pathways.
See the main publication for interpretation of the functional patterns
As a validation, we also used AADDER (bundled with MEGAN6) - a tool which infers functional characteristics based on the annotations of a MALT/MEGAN reference database.
This uses .gff files to compare taxonomic assignments with annotations, which
we downloaded above. A list of genomes used in this list
can be seen in 06-additional_data_files under Data R10.
We ran this against the custom RefSeq genomes we built above both with MALT (fasta files) and AADDER (gff files).
We then ran MALT but instead of immediately producing RMA6 files, we generated SAM files. This was performed by running the following:
02-scripts.backup/008-malt-genbank-refseq_bacarchhomo_gcs_20181122_4step_85pc_supp_0.01 \
04-analysis/screening/malt/temp_input/*/*.fq.gz \
04-analysis/screening/malt/refseq_bacarchhomo_gcs_20181122/To extract the summary statistics for the Refseq runs, we can ran the following on the MALT logs.
grep -e "Loading MEGAN File:" \
-e "Total reads:" \
-e "With hits:" \
-e "Alignments:" \
-e "Assig. Taxonomy" \
-e "Min-supp. changes" \
-e "Numb. Tax. classes:" \
-e "Class. Taxonomy:" \
-e "Num. of queries:" \
-e "Aligned queries:" \
-e "Num. alignments:" \
-e "MinSupport set to:" \
04-analysis/screening/malt/refseq_bacarchhomo_gcs_20181122/*log | cut -d":" -f 2-99 > 00-documentation.backup/99-maltAlignedReadsSummary_raw_refseq_bacarchhomo_gcs_20181122_$(date "+%Y%m%d").txtThe corresponding .megan, .nwk and OTU tables at various taxonomic
levels (as exported by MEGAN) can be seen in 06-additional_data_files under
Data R11.
Then we ran AADDER
aadder-run \
-i 04-analysis/screening/aadder/input/*.sam.gz \
-d 01-data/databases/aadder/ \
-o 04-analysis/screening/aadder/output/ \
-v
pigz -p 112 04-analysis/screening/aadder/output/*
⚠️ AADDERR output files are not provided here due to large size
Finally we ran blast2rma to make the SAM files loadable into MEGAN
blast2rma \
--format SAM \
-i 04-analysis/screening/aadder/output/*out.gz \
-o 04-analysis/screening/aadder/rma6_2/ \
-a2seed 01-data/databases/acc2seed/acc2seed-May2015XX.abin \
-a2t 01-data/databases/acc2tax/nucl_acc2tax-Nov2018.abin \
-vOnce completed, the resulting RMA6 files were opened in MEGAN6CE (v6.15.2) via
MEGAN SERVER, opened in compare mode using absolute counts and 'ignore all
unassigned reads'. I then switched to the 'SEED' categories, 'uncollapse-all',
selected everything, then exported the table as TSV with seedPath_to_count. An
additional file that also included all unassigned reads was also exported (with
the same name but ending in _summarised_Not_Assigned.txt), to allow comparison
between the host genera, the percent of reads that could be assigned functions.
After performing PCA on the protein-level SEED assignments, we wanted to explore
the contribution of each microbial species to the proteins with strongest
loadings in PC1 and PC2, to see if they differed between host genera. To do
this, we opened all of the AADDER .rma6 files from each host genera in MEGAN as
a group (all Homo at once, only ancient Homo all at once, all Gorilla at
once, etc.). From the SEED categories, each protein in the top 10 PC1 and PC2
positive and negative loadings were individually selected, and exported to a new
document. The new document summed the total read counts from all host samples
for each microbial species, so the individual read counts per species per sample
was lost. The species list and read count for ech host genus was exported from
MEGAN as a tsv (and stored in 04-analysis/screening/aadder/tables/).
We manually added an additional 6 columns with a text editor Find and Replace function: Host_Genus, Protein, PC1 code, PC2 code, PC1nomodcode, and PC2nomodcode. The PC<number>code columns indicate the order of proteins from strongest loading (1) to lowest loading (10) of the top 10 strongest loadings in PC1 and PC2, for positive values and negative values. The PC<number>nomodcode columns indicate the same loading order, but for the PCAs that excluded modern Homo samples. For example, pc1n3 is the protein with the 3rd strongest negative loading in PC1, and pc1p3 is the protein with the 3rd strongest positive loading in PC1. These codes were used to make the protein names manageable and consistent in R, which had trouble with the special characters in several protein names.
All analysis of AADDER functional profiles can be found in the R markdown
document here 02-scripts.backup/148-imv-aadder_evolution_function_cleaned.Rmd.
The differences in the number of read assignments to any SEED category, and specifically to the SEED Carbohydrate category, between sample groups were explored.
 Figure R52 | SEED category statistics at different pathway levels are reported by AADDER. a Proportion of total reads assigned to a SEED category by AADDER. b Proportion of reads assigned to the SEED Carbohydrates category at all levels, excluding outlier samples. c Proportion of reads assigned to an enzyme in the SEED Carbohydrate category, excluding outlier samples. d Total number of enzymes in the Carbohydrate category in each sample. e Total abundance (number of reads) of enzymes in the Carbohydrate category in each sample. Note the different scale in each panel. * P < 0.05, ** P < 0.01, *** P<0.001.
Figure R52 | SEED category statistics at different pathway levels are reported by AADDER. a Proportion of total reads assigned to a SEED category by AADDER. b Proportion of reads assigned to the SEED Carbohydrates category at all levels, excluding outlier samples. c Proportion of reads assigned to an enzyme in the SEED Carbohydrate category, excluding outlier samples. d Total number of enzymes in the Carbohydrate category in each sample. e Total abundance (number of reads) of enzymes in the Carbohydrate category in each sample. Note the different scale in each panel. * P < 0.05, ** P < 0.01, *** P<0.001.
We used protein abundance data to perform PCAs, which were plotted to visualize the relationships between samples and controls, as well as within and between samples. The samples that plotted with controls, except plaque, were removed as outliers with poor preservation (File R37).
 Figure R53 | SEED-based enzyme composition of calculus samples clusters host genera distinctly. a PCA with all samples and all enzymes. b PCA with decontam-identified enzymes removed and outlier samples removed. c PCA of oral samples (plaque and calculus) with decontam-identified enzymes and outlier samples removed. d PCA of calculus samples with decontamination-identified enzymes and outlier samples removed.
Figure R53 | SEED-based enzyme composition of calculus samples clusters host genera distinctly. a PCA with all samples and all enzymes. b PCA with decontam-identified enzymes removed and outlier samples removed. c PCA of oral samples (plaque and calculus) with decontam-identified enzymes and outlier samples removed. d PCA of calculus samples with decontamination-identified enzymes and outlier samples removed.
Biplots were used to visualize the protein with strongest loadings in PC1 and PC2, to understand what drove separation of sample groups. The table with these loadings is File R38.
 Figure R54 | PCA biplots with the proteins with the top 10 positive and negative loadings on PC1 and PC2 of SEED protein-level entries identified by AADDER. a Top loadings of PC1 including modern day humans. b Top loadings of PC1 excluding modern day humans. c Top loadings of PC2 including modern day humans. d Top loadings of PC2 excluding modern day humans. Bold protein names indicate these proteins remained in the top 10 when removing modern day humans from analysis. Note in panels B and D (without modern Homo samples) that the y-axis has been reversed to maintain orientation with the other PCAs.
Figure R54 | PCA biplots with the proteins with the top 10 positive and negative loadings on PC1 and PC2 of SEED protein-level entries identified by AADDER. a Top loadings of PC1 including modern day humans. b Top loadings of PC1 excluding modern day humans. c Top loadings of PC2 including modern day humans. d Top loadings of PC2 excluding modern day humans. Bold protein names indicate these proteins remained in the top 10 when removing modern day humans from analysis. Note in panels B and D (without modern Homo samples) that the y-axis has been reversed to maintain orientation with the other PCAs.
The abundance of each of the proteins plotted in the biplots above were visualized in heat maps to visualize the difference between host groups, including modern Homo samples.
 Figure R55 | Heat maps showing the centered log ratio-transformed abundance of the top 10 proteins with strongest loadings from the PCA including modern Homo. Host genus is indicated by the colored bars to the right, where blue is Alouatta, purple is Gorilla, green is Pan, and orange is Homo. Symbols in Homo indicate different groups, where triangle pointed up is Neanderthal, square is pre-agricultural human, circle is pre-antibiotic human, and triangle pointed down is modern day human. a PC1 negative top 10 proteins with strongest loadings. b PC1 positive top 10 proteins with strongest loadings. c PC2 negative top 10 proteins with strongest loadings. d PC2 positive top 10 proteins with strongest loadings.
Figure R55 | Heat maps showing the centered log ratio-transformed abundance of the top 10 proteins with strongest loadings from the PCA including modern Homo. Host genus is indicated by the colored bars to the right, where blue is Alouatta, purple is Gorilla, green is Pan, and orange is Homo. Symbols in Homo indicate different groups, where triangle pointed up is Neanderthal, square is pre-agricultural human, circle is pre-antibiotic human, and triangle pointed down is modern day human. a PC1 negative top 10 proteins with strongest loadings. b PC1 positive top 10 proteins with strongest loadings. c PC2 negative top 10 proteins with strongest loadings. d PC2 positive top 10 proteins with strongest loadings.
The abundance of each of the proteins plotted in the biplots above were visualized in heat maps to visualize the difference between host groups, excluding modern Homo samples.
 Figure R56 | Heat maps showing the centered log ratio-transformed abundance of the top 10 proteins with strongest loadings from the PCA excluding modern Homo. Host genus is indicated by the colored bars to the right, where blue is Alouatta, purple is Gorilla, green is Pan, and orange is Homo. Symbols in Homo indicate different groups, where triangle pointed up is Neanderthal, square is pre-agricultural human, and circle is pre-antibiotic human. a PC1 negative top 10 proteins with strongest loadings. b PC1 positive top 10 proteins with strongest loadings. c PC2 negative top 10 proteins with strongest loadings. d PC2 positive top 10 proteins with strongest loadings.
Figure R56 | Heat maps showing the centered log ratio-transformed abundance of the top 10 proteins with strongest loadings from the PCA excluding modern Homo. Host genus is indicated by the colored bars to the right, where blue is Alouatta, purple is Gorilla, green is Pan, and orange is Homo. Symbols in Homo indicate different groups, where triangle pointed up is Neanderthal, square is pre-agricultural human, and circle is pre-antibiotic human. a PC1 negative top 10 proteins with strongest loadings. b PC1 positive top 10 proteins with strongest loadings. c PC2 negative top 10 proteins with strongest loadings. d PC2 positive top 10 proteins with strongest loadings.
We looked at whether specific species contributed the proteins with strongest loadings in the biplots above to look for host-specific microbial species contributions to proteins. First we looked at the proteins in strongest negative loadings in PC1.
 Figure R57 | Species contributions to proteins with the strongest negative loadings in PC1. Bar graphs of the species that contribute >15% to the proteins with strongest negative loadings in PC1. a Including present-day Homo samples, grouped by protein. Symbols indicate the same proteins in panel C. b Including present-day Homo samples, grouped by host genus. c Excluding present-day Homo samples, grouped by protein. Symbols indicate the same proteins in panel A. d Excluding present-day Homo samples, grouped by host genus. All genera that individually contributed <15% are grouped together as Other. Protein names correspond to the numbers in the tables of Figure R53, where pc1/pc2 indicate the component, p/n indicate positive/negative, and the final number indicates the protein. Note different colors for panels A/B and C/D.
Figure R57 | Species contributions to proteins with the strongest negative loadings in PC1. Bar graphs of the species that contribute >15% to the proteins with strongest negative loadings in PC1. a Including present-day Homo samples, grouped by protein. Symbols indicate the same proteins in panel C. b Including present-day Homo samples, grouped by host genus. c Excluding present-day Homo samples, grouped by protein. Symbols indicate the same proteins in panel A. d Excluding present-day Homo samples, grouped by host genus. All genera that individually contributed <15% are grouped together as Other. Protein names correspond to the numbers in the tables of Figure R53, where pc1/pc2 indicate the component, p/n indicate positive/negative, and the final number indicates the protein. Note different colors for panels A/B and C/D.
We looked at whether specific species contributed the proteins with strongest loadings in the biplots above to look for host-specific microbial species contributions to proteins. Then we looked at the proteins in strongest positive loadings in PC1.
 Figure R58 | Microbial genus contributions to proteins with the strongest positive loadings in PC1. Bar graphs of the species that contribute >15% to the proteins with strongest positive loadings in PC1. a Including present-day Homo grouped by protein. Symbols indicate the same proteins in panel C. b grouped by host genus, c excluding present-day Homo grouped by protein. Symbols indicate the same proteins in panel A. d with no present-day humans grouped by host genus. All genera that individually contributed <15% are grouped together as Other. Protein names correspond to the numbers in the tables of Figure R53, where pc1/pc2 indicate the component, p/n indicate positive/negative, and the final number indicates the protein. Note different colors for panels A/B and C/D.
Figure R58 | Microbial genus contributions to proteins with the strongest positive loadings in PC1. Bar graphs of the species that contribute >15% to the proteins with strongest positive loadings in PC1. a Including present-day Homo grouped by protein. Symbols indicate the same proteins in panel C. b grouped by host genus, c excluding present-day Homo grouped by protein. Symbols indicate the same proteins in panel A. d with no present-day humans grouped by host genus. All genera that individually contributed <15% are grouped together as Other. Protein names correspond to the numbers in the tables of Figure R53, where pc1/pc2 indicate the component, p/n indicate positive/negative, and the final number indicates the protein. Note different colors for panels A/B and C/D.
We looked at whether specific species contributed the proteins with strongest loadings in the biplots above to look for host-specific microbial species contributions to proteins. Then we looked at the proteins in strongest PC2 loadings characterizing non-human primates.
 Figure R59 | Species contributions to proteins with the strongest PC2 loadings in the direction characterizing Alouatta/Gorilla/Pan. Bar graphs of the genera, summed from species, which contribute >15% to the proteins with strongest positive loadings in PC2. a Including present-day Homo samples, grouped by protein. Symbols indicate the same proteins in panel C. b Including present-day Homo samples, grouped by host genus. c Excluding present-day Homo samples, grouped by protein. Symbols indicate the same proteins in panel A. d Excluding present-day Homo samples, grouped by host genus. All genera that individually contributed <15% are grouped together as Other. Protein names correspond to the numbers in the tables of Figure R53, where pc1/pc2 indicate the component, p/n indicate positive/negative, and the final number indicates the protein. Note different colors for panels A/B and C/D.
Figure R59 | Species contributions to proteins with the strongest PC2 loadings in the direction characterizing Alouatta/Gorilla/Pan. Bar graphs of the genera, summed from species, which contribute >15% to the proteins with strongest positive loadings in PC2. a Including present-day Homo samples, grouped by protein. Symbols indicate the same proteins in panel C. b Including present-day Homo samples, grouped by host genus. c Excluding present-day Homo samples, grouped by protein. Symbols indicate the same proteins in panel A. d Excluding present-day Homo samples, grouped by host genus. All genera that individually contributed <15% are grouped together as Other. Protein names correspond to the numbers in the tables of Figure R53, where pc1/pc2 indicate the component, p/n indicate positive/negative, and the final number indicates the protein. Note different colors for panels A/B and C/D.
We looked at whether specific species contributed the proteins with strongest loadings in the biplots above to look for host-specific microbial species contributions to proteins. Last we looked at the proteins in strongest PC2 loadings characterizing Homo.
 Figure R60 | Microbial genus contributions to proteins with the strongest PC2 loadings in the direction characterizing Homo. Bar graphs of the genera, summed from species, that contribute >15% to the proteins with strongest negative loadings in PC2. a Including present-day Homo samples, grouped by enzyme. Symbols indicate the same proteins in panel C. b Including present-day Homo samples, grouped by host genus. c Excluding present-day Homo samples, grouped by enzyme. Symbols indicate the same proteins in panel A. d Excluding present-day Homo samples, grouped by host genus. All genera that individually contributed <15% are grouped together as Other. Protein names correspond to the numbers in the tables of Figure R53, where pc1/pc2 indicate the component, p/n indicate positive/negative, and the final number indicates the protein. Note different colors for panels A/B and C/D.
Figure R60 | Microbial genus contributions to proteins with the strongest PC2 loadings in the direction characterizing Homo. Bar graphs of the genera, summed from species, that contribute >15% to the proteins with strongest negative loadings in PC2. a Including present-day Homo samples, grouped by enzyme. Symbols indicate the same proteins in panel C. b Including present-day Homo samples, grouped by host genus. c Excluding present-day Homo samples, grouped by enzyme. Symbols indicate the same proteins in panel A. d Excluding present-day Homo samples, grouped by host genus. All genera that individually contributed <15% are grouped together as Other. Protein names correspond to the numbers in the tables of Figure R53, where pc1/pc2 indicate the component, p/n indicate positive/negative, and the final number indicates the protein. Note different colors for panels A/B and C/D.
Lastly, we looked at whether proteins in specific metabolic pathways were as distinct between host groups as all proteins combined, by performing and plotting PCAs with proteins from only the Carbohydrate, Amino acid, and Fatty acid metabolic pathways independently.
 Figure R61 | PCAs using SEED-classified proteins belonging to specific metabolic pathway categories. Proteins in pathways that process major biomolecules do not each separate samples by host genus in a pattern similar to that seen in taxonomy, and all proteins. a Only proteins in the Carbohydrates category separate the samples by host genera in a pattern similar to that seen for taxonomy and all proteins. b Amino acid category proteins do not separate samples by host genera as distinctly as Carbohydrate category proteins. c Proteins in the Fatty acids, Lipids, and Isoprenoids category do not separate samples by host genera as distinctly as Carbohydrate category proteins.
Figure R61 | PCAs using SEED-classified proteins belonging to specific metabolic pathway categories. Proteins in pathways that process major biomolecules do not each separate samples by host genus in a pattern similar to that seen in taxonomy, and all proteins. a Only proteins in the Carbohydrates category separate the samples by host genera in a pattern similar to that seen for taxonomy and all proteins. b Amino acid category proteins do not separate samples by host genera as distinctly as Carbohydrate category proteins. c Proteins in the Fatty acids, Lipids, and Isoprenoids category do not separate samples by host genera as distinctly as Carbohydrate category proteins.
See main publication for discussion of these results.
Finally, we wanted to see how many genes identified in the samples were identified by both HUMAnN2 and AADDER, within major biomolecule processing pathways (Amino acids, Carbohydrates, Fatty acids/Lipids).
Both the HUMAnN2 KEGG orthologs and the AADDER SEED proteins include the Enzyme Commission number (EC number) on a majority of the orthologs/proteins they report, so we compared the EC numbers between the two programs.
For HUMAnN2, all orthologs that are included in the KEGG Metabolism Pathways
'Amino acid', 'Carbohydrate', and 'Lipid' were individually selected out of the
full HUMAnN2 ortholog list. For AADDER, all proteins in the pathways
Amino Acids, Carbohydrates, and Fatty Acids, Lipids, and Isoprenoids were
selected out of the full AADDER table. All analyses based on EC numbers can be
found in the R markdown document here
02-scripts.backup/149-imv-aa-carbs-lipids_kegg-vs-seed.Rmd.