Strainy is a tool for phasing and assembly of bacterial strains from long-read sequencing data (either Oxford Nanopore or PacBio). Given a reference (or collapsed de novo assembly) and set of aligned reads as input, Strainy produces multi-allelic phasing, individual strain haplotypes and strain-specific variant calls.
Compared to the current metagenomic strain profiling approaches, Strainy offers multiple unique features. First, it provides direct phasing of strains-specific variants, enabling evolutionary analysis of haplotypes rather than individual mutations. Second, Strainy provides a method to quantity strains and distinguish the most abundant and/or most divergent strains. Third, Strainy enables assembly-based analysis, which is useful in the absence of a high quality reference genome, especially in cross-samples comparison.
- Installation
- Quick Usage
- Docker container
- Strainy input
- Preparing de novo metagenomic assemblies
- Parameters description
- Output Files
- Strainy tutorial
- Overview of the Strainy algorithm
The recommended way of installing is through conda or mamba . Strainy is distributed via bioconda channel and can be installed using one of the following:
conda install bioconda::strainy
or
mamba install strainy
The other way is to install from the repository:
git clone https://github.com/katerinakazantseva/strainy
cd strainy
git submodule update --init
make -C submodules/Flye
conda env create -f environment.yml -n strainy
Once installed, you will need to activate the conda environment prior to running:
conda activate strainy
./strainy.py -h
Note that if you use an M1 conda installation, you should run conda config --add subdirs osx-64 before installation.
Find details here
After repository cloning/installation, you should be able to run:
conda activate strainy
./strainy.py --gfa_ref test_set/toy.gfa --fastq test_set/toy.fastq.gz --output out_strainy --mode hifi --threads 8
The --gfa specifices input strain-collapsed graph (e.g. from de novo metagenomic assembly). --fastq
specifies the matching long-read data. --output is the output directory, --mode speficies read type (either hifi or nano),
--threads specifices the number of threads o use.
The output directory out_strainy will contain strain-level assmebly strain_contigs.gfa along with
phased alignment alignment_phased.bam, strain variants calls strain_variants.vcf and other info.
See below for the details on how to interpret strainy output.
Also see a more detailed tutorial for the example of how to use Strainy.
Alternatively, you can use a Docker container (using the example provided in test_set Strainy directory):
ST_DIR=`pwd`
docker run -v $ST_DIR:$ST_DIR -u `id -u`:`id -g` mkolmogo/strainy:1.0 strainy --gfa $ST_DIR/test_set/toy.gfa --fastq $ST_DIR/test_set/toy.fastq.gz -o $ST_DIR/out_strainy --threads 8 --mode hifi
Strainy supports PacBio HiFi, Nanopore R9 (Guppy5+) and R10 sequencing.
The two main inputs to Strainy are:
-
GFA fileA collapsed de novo metagenomic assembly that can be produced with metaFlye. For metaFlye parameters, please see Preparing de novo metagenomic assemblies. Alternatively, a reference in fasta format could be provided as input (--fasta_ref). -
Reads (fasta/fastq)containing reads that need to be assmebled / phased. In the case of improving collapsed de novo assembly, same reads should be used for the assembler input. -
Optinal alignment / variant calls. A user can provide their own alignment inbamformat and variant calls invcfformat (that will be used for phasing). If these files are provided, splitting of long unitigs must be disabled by adding--unitig-split-length 0. It is recommended to split long sequences prior to producing alignment / variant calls, for example using the Strainy--only-splitoption. Long unitigs (50 kb+) may significantly slow down Strainy.
We have developed Strainy using metaFlye metagenomic assembly graphs as input. The recommended
set of parameters is --meta --keep-haplotypes --no-alt-contigs -i 0.
Note that -i 0 disables metaFlye's polishing procedure, which we found to improve read assignment
to bubble branches during minimap2 realignment. --keep-haplotypes retains structural
variations between strains on the assembly graph. --no-alt-contigs disables the output of
"alternative" contigs, which can later confuse the read aligner.
| Argument | Description |
|---|---|
| -o, --output | Output directory |
| -g, --gfa_ref | Input assembly graph (.gfa) (may be produced with metaFlye or minigraph) |
| -f, --fasta_ref | Input reference fasta (for linear genome) |
| -q, --fastq | FASTQ file containing reads ( PacBio HiFi or Nanopore sequencing) |
| -m, --mode | Type of the reads {hifi,nano} |
| Argument | Description |
|---|---|
| --snp | .vcf file, with variants of the desired allele frequency. If not provided, Strainy will use the built-in pileup-based caller |
| -b, --bam | .bam file generated by aligning the input reads to the input graph, minimap2 will be used to generate a .bam file if not provided |
| -a, --allele-frequency | Allele frequency threshold for built-in pileup-based caller. Will only work if --snp is not used (default: None) |
| -d, --cluster-divergence | The maximum number of total mismatches allowed in the cluster per 1 kbp. Should be selected depending on SNP rates and their accuracy. Higher values can reduce high fragmentation at the cost of clustering accuracy (default: None) |
| --unitig-split-length | The length (in kb) which the unitigs that are longer will be split, set 0 to disable (default: 50 kb) |
| --min-unitig-coverage | The minimum coverage threshold for phasing unitigs, unitigs with lower coverage will not be phased (default: 20) |
| --max-unitig-coverage | The maximum coverage threshold for phasing unitigs, unitigs with higher coverage will not be phased (default: 500) |
| -t, --threads | Number of threads to use (default: 4) |
| --debug | Enables debug mode for extra logs and output |
| -s, --stage | Stage to run: phase, transform or e2e (phase + transform) (default: e2e) |
-
alignment_phased.bamPhased input alignemnt. Phased is defined viaYCtag, which could also be used for IGV visualization. The matching reference could be found atpreprocessing_data/gfa_converted.fasta. If (unphased) alignment was not provided as input, Strainy produced alignment against the input gfa using minimap2. -
strain_unitigs.gfaTranformed graph that incorporates assmebled strain haplotypes. These are "finer" strain unitigs that match the CSV tables with additional info below. -
strain_contigs.gfaA simplified and extended version of teh graph after final simplification. Some strain unitigs are joined into longer contigs, so these sequenced may no longer have IDs matching to the CSV tables below. -
strain_variants.vcfA file with variant produced from assembled strain haplotypes in VCF format. In the INFO field,ALT_HAPrefers to strain unitigs that support the ALT version of the variant, andREF_HAPcorrespond to the list of unitigs that contain no variant (reference state). -
reference_unitig_info_table.csvAdditional statistics for reference sequences (such as length, coverage, SNP rate). -
phased_unitig_info_table.csvStatistics for each phased strain unitig (matching thestrain_unitigs.gfafile). For each strain unitig, its length, coverage, SNP rate and other statistics are reported. -
multiplicity_stats.txtDataset-level summary of strain multiplicity and strain divergence, along with other assembly-based statistics.
Here we illustrate Strainy usage scenario using the simulated metagenomic dataset of five E. coli strains. Download the input data:
wget https://zenodo.org/records/11187925/files/strainy_ecoli_example.tar.gz
tar -xvf strainy_ecoli_example.tar.gz
It contains simulated reads and metaFlye assembly graph. Optionally, if you want to reproduce the metaFlye assembly, you can run:
flye --nano-hq strainy_ecoli_example/ecoli_5strain_sim_badread.fastq.gz -o metaflye -t 30 --meta --no-alt-contigs --keep-haplotypes -i 0
Then, you can run Strainy using:
./strainy.py --gfa_ref strainy_ecoli_example/ecoli_5strain_metaflye_hap.gfa --fastq strainy_ecoli_example/ecoli_5strain_sim_badread.fastq.gz --mode nano -t 30 --output strainy_out
This run may take ~2h in 30 threads. If you don't want to wait, you can download results from here:
wget https://zenodo.org/records/11187925/files/strainy_ecoli_out.tar.gz
tar -xvf strainy_ecoli_out.tar.gz
Now, let's take a look at the results! multiplicity_info.txt will contain some assembly stats, and the information about strain multiplicity:
Reference utgs input: len: 6459094 num: 515 N50:28856
Reference utgs select: len: 6367432 num: 433 N50:29273
Reference utgs phased: len: 5439417 num: 319 N50:31133
Strain utgs asmembled: len: 20620769 num: 1729 N50:16393
Multiplicity
Mul RefSeqLength
1 1653400 ********************
2 455200 *****
3 770300 *********
4 1634900 *******************
5 1547500 ******************
6 246800 **
7 21700
8 8300
10 1800
The total input size was ~6.4Mb, and Strainy transformed it into 20.6 Mb of strain-specific sequence. ~1.6 of the input reference strain had multiplicity 1 - e.g. not collapsed, but the rest corresponded to 2+ strains. Most collapsed sequence had either 4 or 5. The bottom of the file also contains info about the divergence of the assembled strain (wrt to the collapsed reference):
SNP divergence
Rate StrainSeq
0.03981 371500 **
0.03162 1099100 *******
0.02512 1563900 **********
0.01995 1495400 *********
0.01585 2964600 *******************
0.01259 2523100 ****************
0.01000 3052800 ********************
0.00794 1685300 ***********
0.00631 1516200 *********
0.00501 861600 *****
0.00398 586200 ***
0.00316 374300 **
0.00251 301800 *
0.00200 233500 *
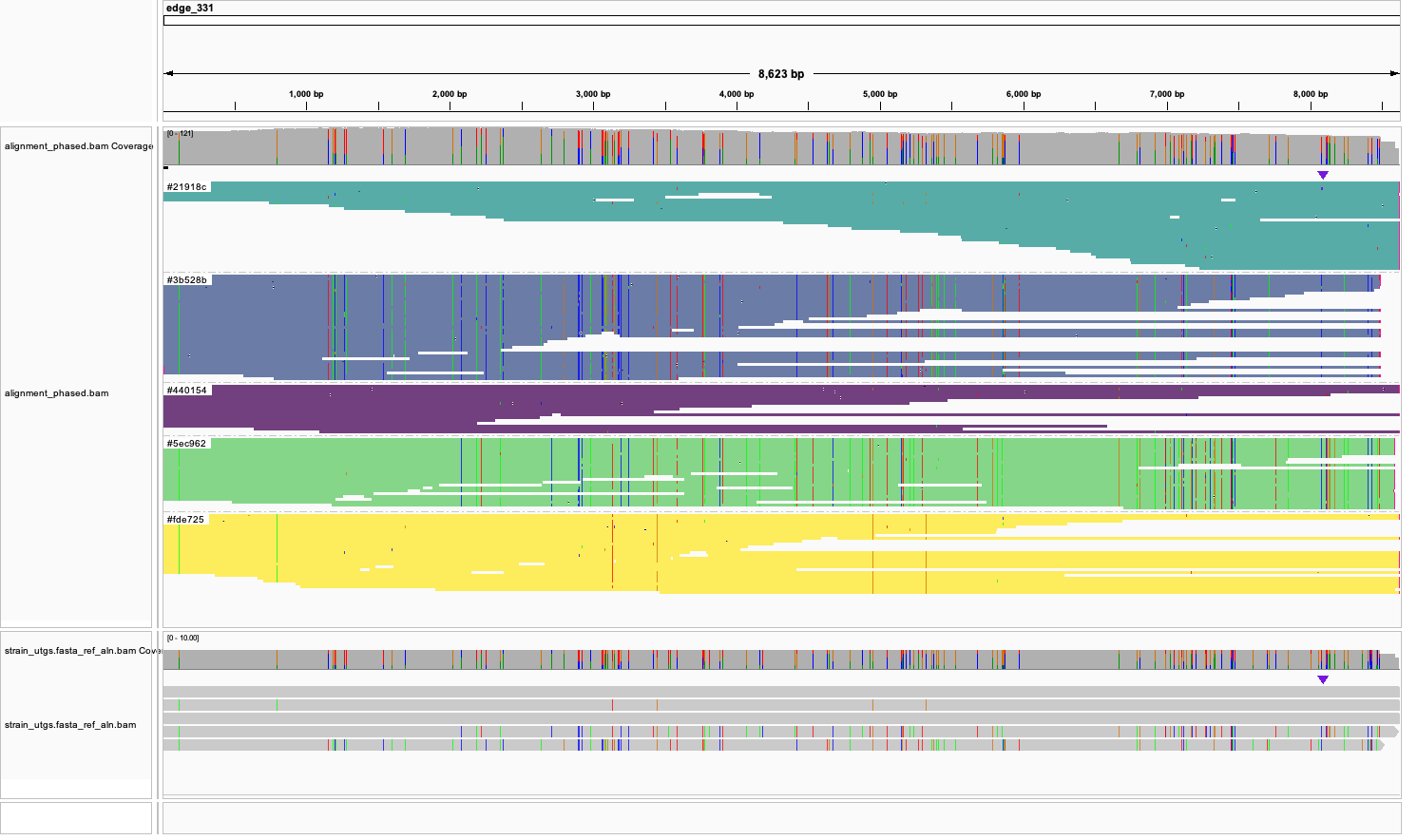
Next, let's use IGV to look in more detail. Open preprocessing_data/gfa_converted.fasta as a reference,
and alignment_phased.bam as a track. This shows the phased alignment, and you can enable coloring and grouping by YC tag.
In addition, you can add intermediate/strain_utgs.fasta_ref_aln.bam track with the assmebled strain haplotgs. It may look
something like this, clearly showing 5 read clusters and assembled haplotypes:
Each variant position will be described in strain_variants.vcf. For example, the following VCF entry tells that the substitution is supported
by 2 strains (enumerated in ALT_HAP), and 3 strains have reference state (enumerated in REF_HAP).
edge_308 3453 Strainy_SNP_17146 G T 60 PASS ALT_HAP=edge_308_119,edge_308_1000107;REF_HAP=edge_308_10015,edge_308_10014,edge_308_20016 GT:DV:D P 0/1:2:5
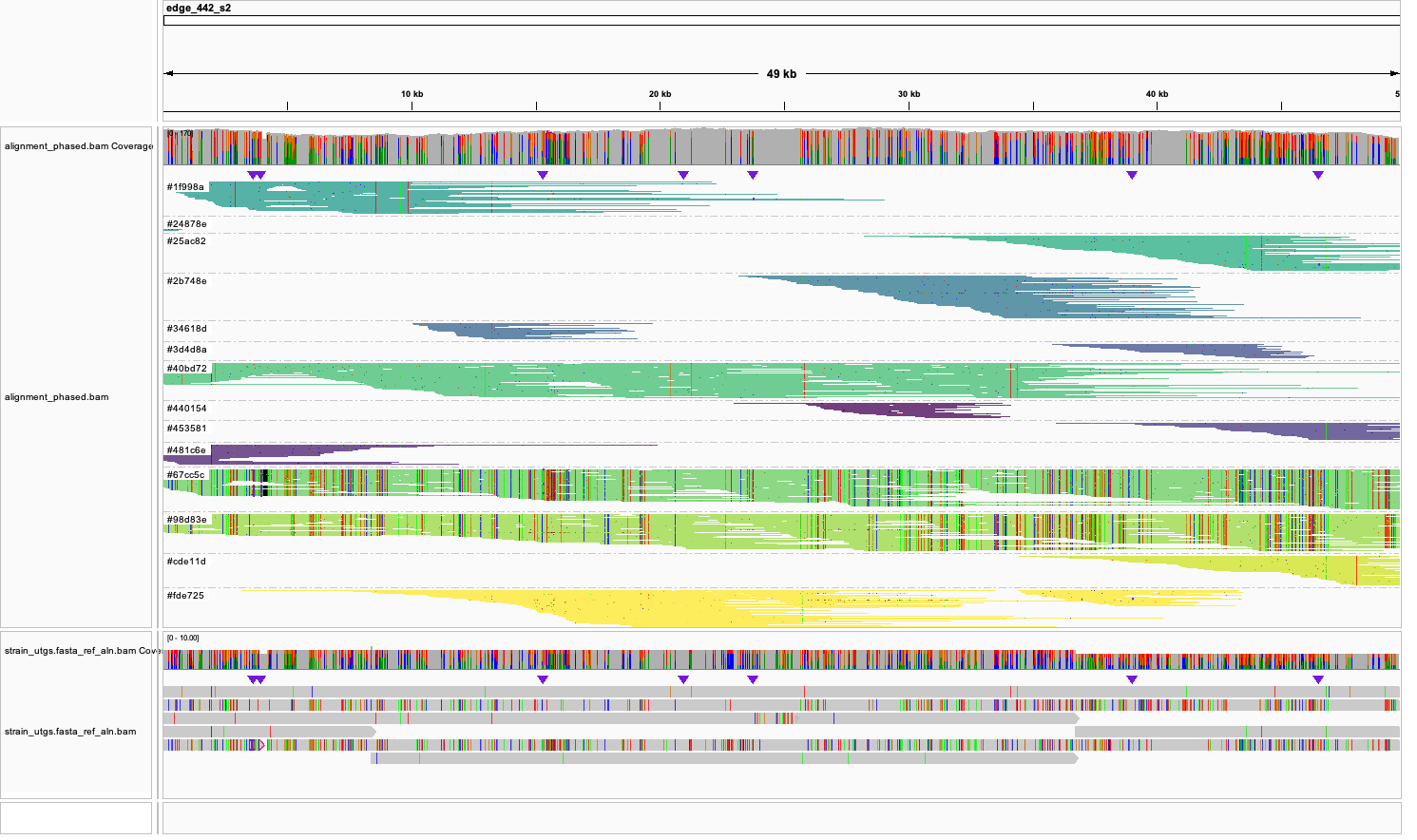
But often, it may look a bit more complex. On the example eblow you see more strain clusters that sometimes do not span the entire reference unitig. This often happens if there is not enough heterozygosity to separate two or more strains:
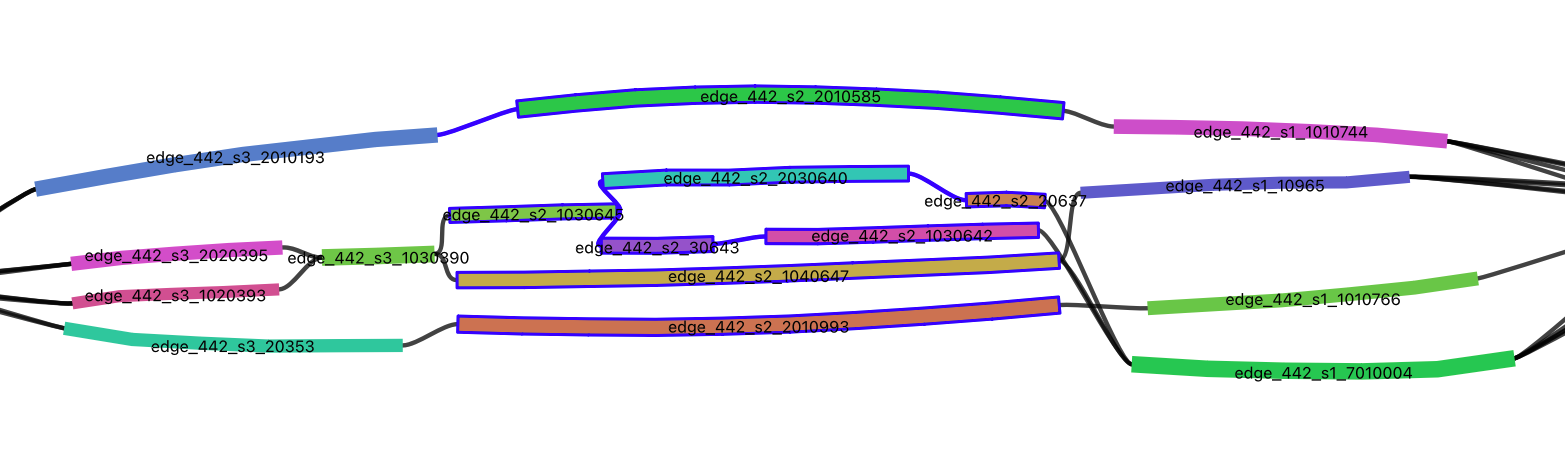
In fact, if you visualize the strain_unitigs.gfa with Bandage, you can see how
these strain unitigs are connected together:
Finally, phased_unitig_info_table.csv contains statistics about individual phased strain unitigs.
This is a brief description, and more details are available in our preprint.
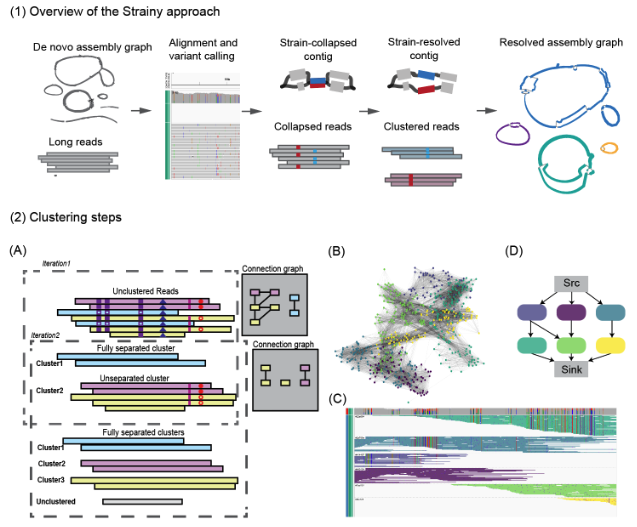
The goal of the Strainy is to recover collapsed strain haplotypes from the input graph. First, input reads are mapped against the input assembly graph and the alignment is used to call SNP variants (which could also be provided as input). Then, the phasing module is used to group aligned reads into strain clusters. Afterwards, strain haplotypes are reassembled from clustered reads.
In brief, the phasing module works as follows. First, for each strain-collapsed input contig, Strainy builds a connection graph, which encodes the pairwise distances between reads aligned to this contig. Next, it clusters reads based on the strain of origin using the community detection approach. While initial clustering separates the most divergent strains, closely related strains (with less variants between them) may remain collapsed. To overcome this, Strainy recursively repeats the clustering procedure with the increased sensitivity to strain variants Clustering stringency threshold may be increased to allow collapsing of very similar strains, while separating more divergent strains.
Clustered reads are then reassembled locally using the Flye polisher, forming strain haplotigs. Strainy builds an overlap graph of these haplotigs, where paths on the graph correspond to phased strain sequence, interleaved by unphased sequences. Finally, Strainy applies graph simplification algorithms to improve the assembly contiguity. As output, Strainy provides assembled strain haplotypes, along with phased read alignment, strain variant calls (small and structural) and information about strain multiplicity and divergence.
Consensus function of Strainy is Flye
Community detection algorithm is Karate club
Strainy was originally developed at at Kolmogorov lab at NCI
Code contributors:
- Ekaterina Kazantseva
- Ataberk Donmez
- Mikhail Kolmogorov
Ekaterina Kazantseva, Ataberk Donmez, Maria Frolova, Mihai Pop, Mikhail Kolmogorov. "Strainy: phasing and assembly of strain haplotypes from long-read metagenome sequencing" Nature Methods 2024, https://doi.org/10.1038/s41592-024-02424-1
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.