EasyQTLseq is a R package for QTL-seq analysis.
EasyQTLseq can be installed from GitHub via devtools:
# install devtools
install.packages("devtools")
# install easyQTLseq
devtools::install_github("laowang1992/easyQTLseq")The VCF file contained parent(s) and two bulks was generated from GATK best practices pipeline. Utilizing VariantsToTable function of GATK to extract GT, AD and GQ information for improving reading and processing speed.
java -Xmx30g -jar ${GATK} \
-R ${genome} -T VariantsToTable \
-F CHROM -F POS -F REF -F ALT -GF GT -GF AD -GF GQ \
-V ${BSA}.filter.SNPs.vcf.gz -o ${BSA}.filter.SNPs.tableIf you only have a vcf file, and you don’t install GATK on your
computer. Then you can read the vcf file using vcfR package, and use
vcf2table() function in easyQTLseq to convert the vcf object to a
table, which is similar the output of GATK’s VariantsToTable. The
output of vcf2table can be used for select_sample_and_SNP() directly.
library(vcfR)
library(easyQTLseq)
file_path <- system.file("extdata", "A07.SNPs.vcf.gz", package = "easyQTLseq")
x <- read.vcfR(file = file_path)
data <- vcf2table(x = x)A sample file is included with this package. There are four samples in this sample file, R3 is the high phenotype parent sample, qY is the low phenotype parent sample, R is the high phenotype bulk sample, and Y is the low phenotype bulk sample.
library(easyQTLseq)
# Example with sample data from a GATK table.
file_path <- system.file("extdata", "subset.table.gz", package = "easyQTLseq")
# readr::read_tsv() has a faster speed than read.table() when reading a file.
data <- readr::read_tsv(file = file_path)## Rows: 515669 Columns: 16
## ── Column specification ────────────────────────────────────────────────────────
## Delimiter: "\t"
## chr (11): CHROM, REF, ALT, R.GT, R.AD, R3.GT, R3.AD, Y.GT, Y.AD, qY.GT, qY.AD
## dbl (5): POS, R.GQ, R3.GQ, Y.GQ, qY.GQ
##
## ℹ Use `spec()` to retrieve the full column specification for this data.
## ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.
Some basic information should be assigned use select_sample_and_SNP().
This function will return a QTLseq S3 object.
x <- select_sample_and_SNP(data = data, highP = "qY", lowP = "R3", highB = "Y", lowB = "R", popType = "F2", bulkSize = c(30, 30))
x## $data
## # A tibble: 128,751 × 12
## CHROM POS REF ALT HP.DP LP.DP HB.HP.AD HB.LP.AD HB.DP LB.HP.AD
## <chr> <dbl> <chr> <chr> <int> <int> <int> <int> <int> <int>
## 1 scaffoldA01 275959 A C 17 24 6 6 12 16
## 2 scaffoldA01 320766 G A 19 11 6 6 12 10
## 3 scaffoldA01 361230 A T 18 6 6 5 11 8
## 4 scaffoldA01 361486 G T 26 9 13 5 18 8
## 5 scaffoldA01 361875 A C 19 5 8 0 8 4
## 6 scaffoldA01 361884 T G 19 5 8 0 8 6
## 7 scaffoldA01 362128 A C 19 6 7 5 12 4
## 8 scaffoldA01 362153 T G 20 5 8 5 13 6
## 9 scaffoldA01 364170 C T 17 14 12 9 21 14
## 10 scaffoldA01 365454 T C 18 10 11 5 16 15
## # ℹ 128,741 more rows
## # ℹ 2 more variables: LB.LP.AD <int>, LB.DP <int>
##
## $highP
## [1] "qY"
##
## $lowP
## [1] "R3"
##
## $highB
## [1] "Y"
##
## $lowB
## [1] "R"
##
## $popType
## [1] "F2"
##
## $bulkSize
## [1] 30 30
##
## $slidwin
## data frame with 0 columns and 0 rows
##
## $chrLen
## # A tibble: 3 × 2
## CHROM Len
## <chr> <dbl>
## 1 scaffoldA01 37932493
## 2 scaffoldA07 32276921
## 3 scaffoldA09 65861795
##
## attr(,"class")
## [1] "QTLseq" "WithParent" "BothParent"
This function can handle different situation, such as both parents of the segregation population are present, only high parent or low parent is present, and no parent is present, or one of these present has a reference genome used for SNP calling.
# If only one parent is present, e.g. high parent.
x_onlyHP <- select_sample_and_SNP(data = data, highP = "qY", highB = "Y", lowB = "R", popType = "F2", bulkSize = c(30, 30))
# If no parent is present.
x_noParent <- select_sample_and_SNP(data = data, highB = "Y", lowB = "R", popType = "F2", bulkSize = c(30, 30))
# If no parent is present, but high parent has a reference genome, this reference genome is used for SNP calling. Then the `highP` parameter should be "REF".
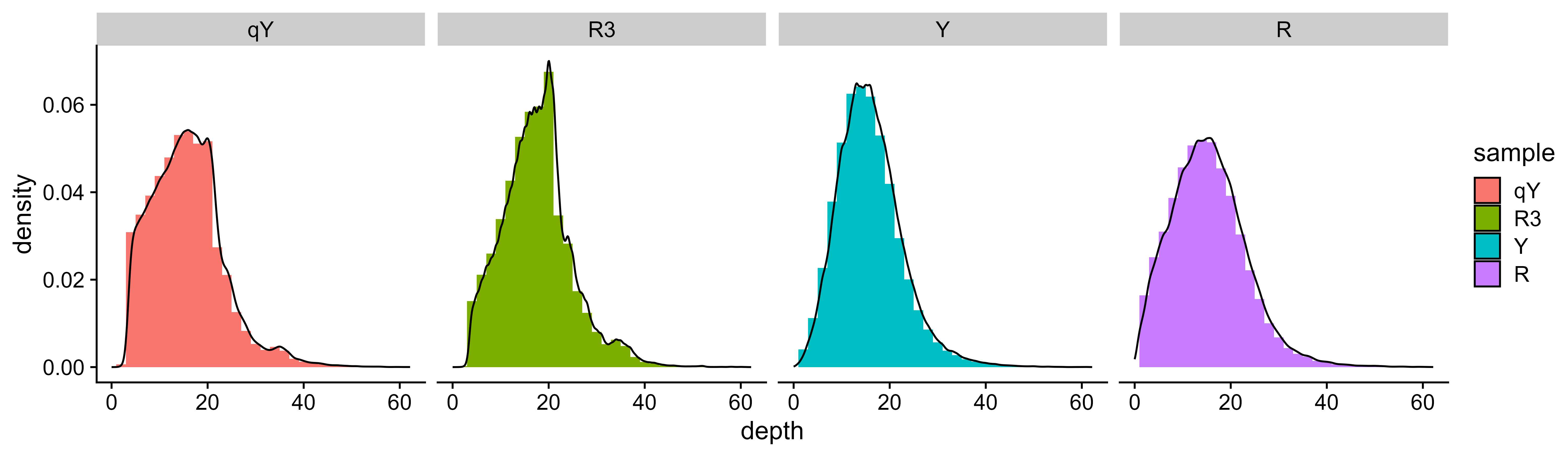
x_HPisREF <- select_sample_and_SNP(data = data, highP = "REF", highB = "Y", lowB = "R", popType = "F2", bulkSize = c(30, 30))After select samples and SNPs, the coverage depth distribution of every
samples is calculated, then a density distribution figure is drawn,
named <outPrefix>.depth_density.pdf|png
depth_statistics(x = x, outPrefix = "outprefix")For low coverage depth SNP may have low reliability and accuracy, and extremely high coverage depth may be derived from repetitive sequence. These SNP should be omited.
# default minimum coverage depth is 6, default maximum coverage depth is `average+3*sd`.
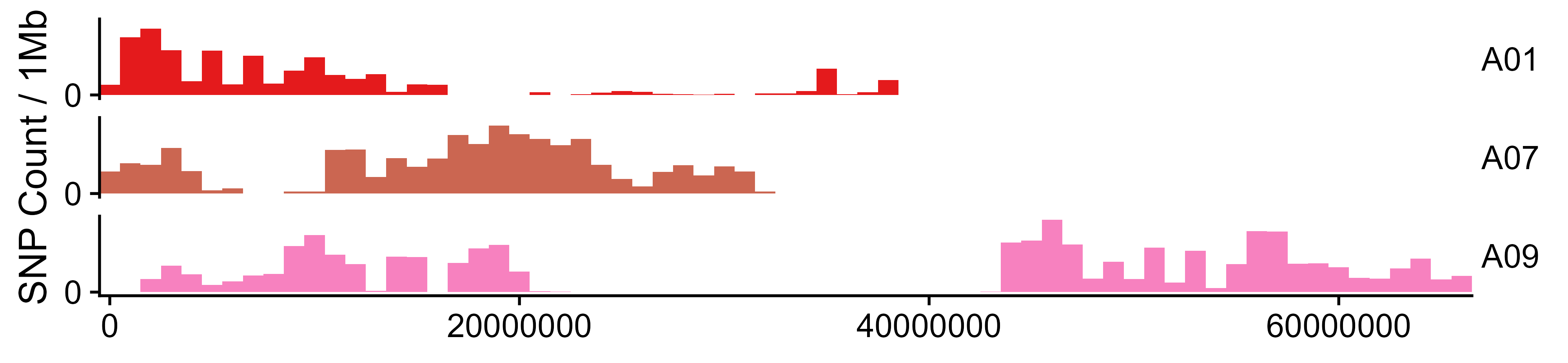
x_filter <- filterDP(x = x)SNPs may be unevenly distributed on chromosomes. This step will show the
distribution of SNP along the chromosome and generate
<outprefix>.SNP_number_per_chr.txt|csv and
<outprefix>.SNP_distribution_histogram.pdf|png.
SNP_distribution(x = x_filter, outPrefix = "outprefix",
targetChr = c("scaffoldA01", "scaffoldA07", "scaffoldA09"),
chrLabel = c("A01", "A07", "A09"))If you want analyze QTL-seq using other method or software, you can
export allele depths information using export_dp(). This information
will be export to <outprefix>.Depth_information.txt|csv in work
directory.
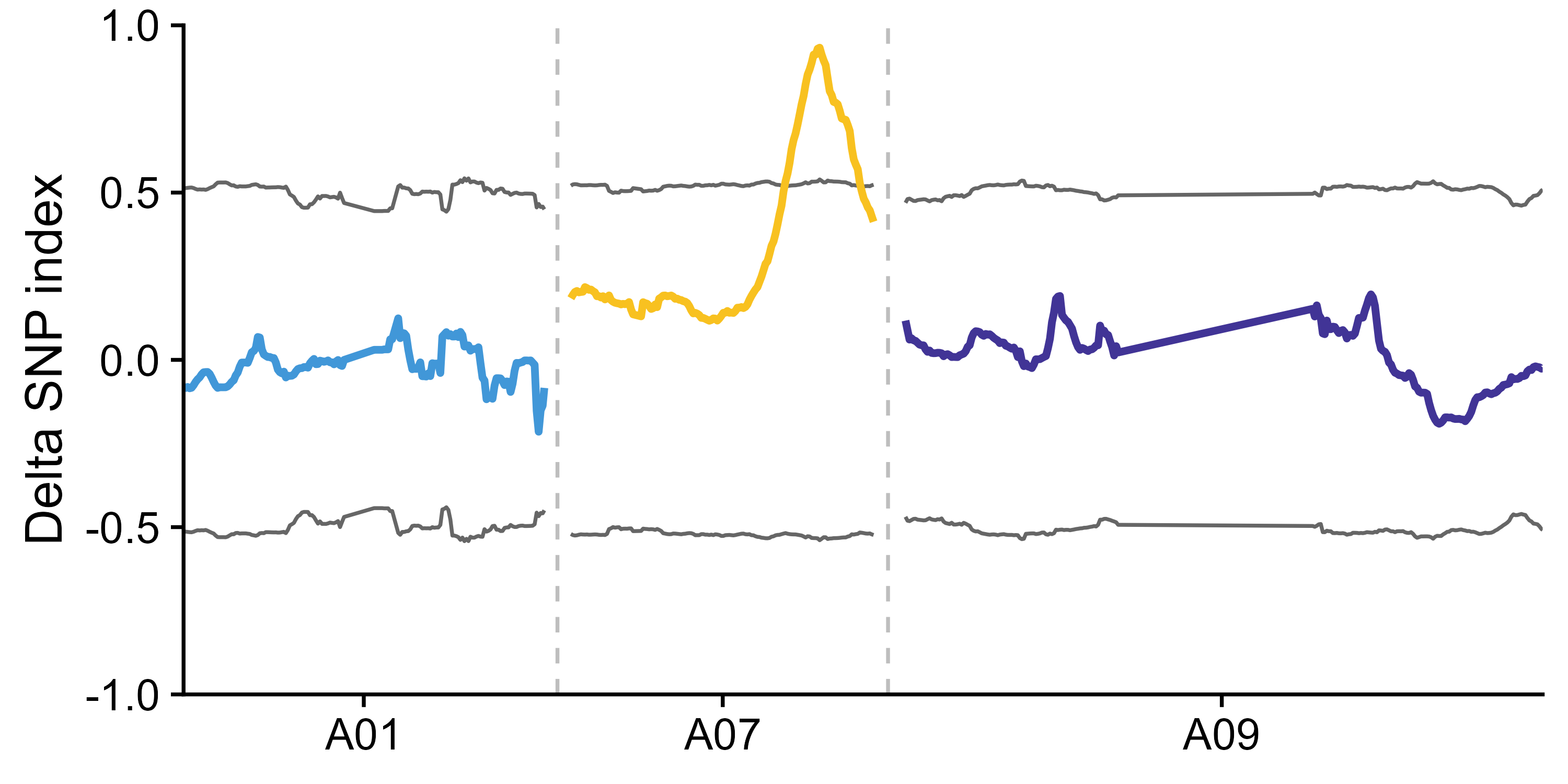
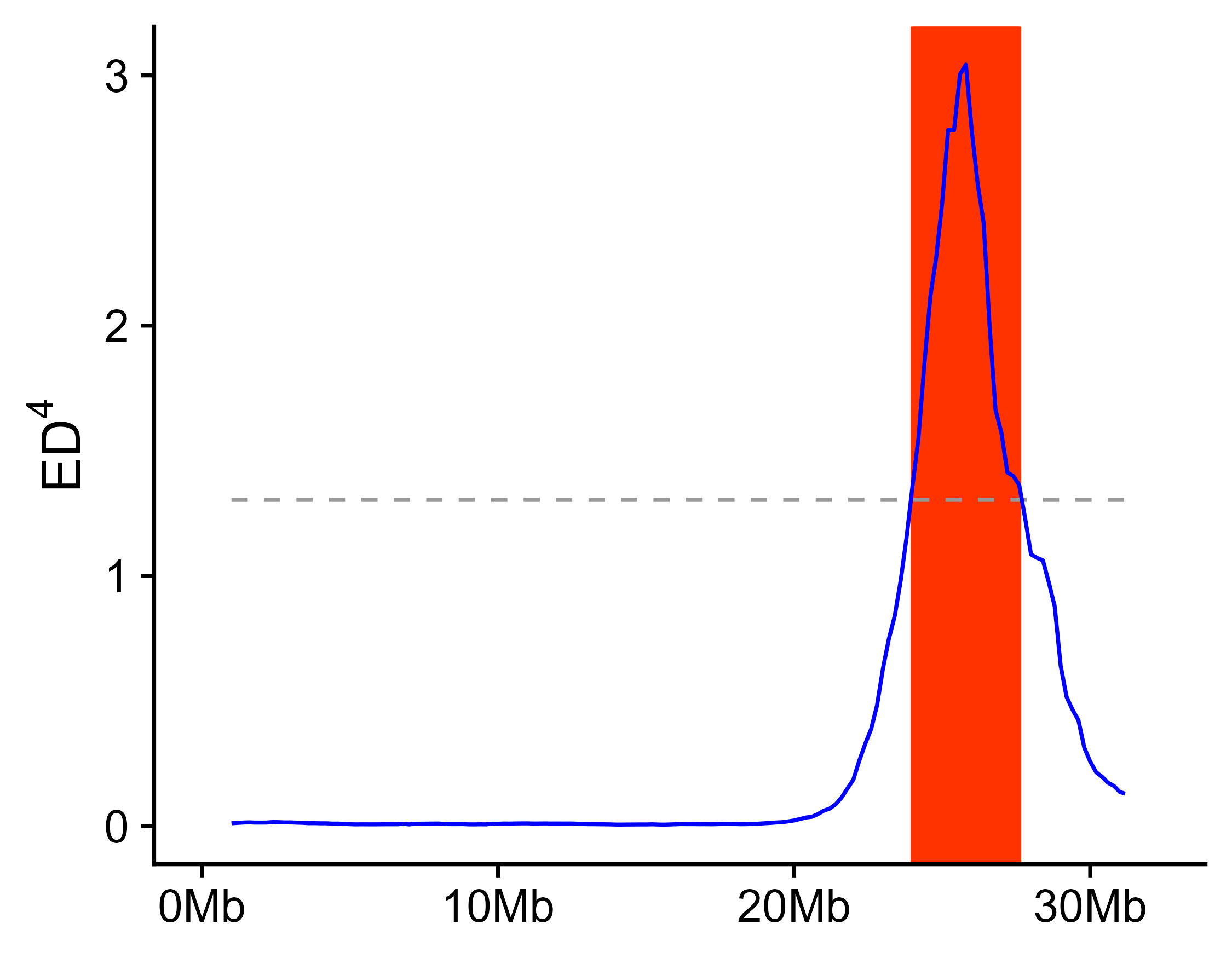
export_dp(x = x_filter, outPrefix = "outprefix")To reduce noise in QTL-seq analsis, a sliding window method is adopted to calculate SNP index, delta SNP index, and Euclidean distance (ED).
If parent is present in the data or parent has a reference genome which is used for SNP calling, both delta SNP index and DE are calculated, if no parent is present in the data, only ED is calculated.
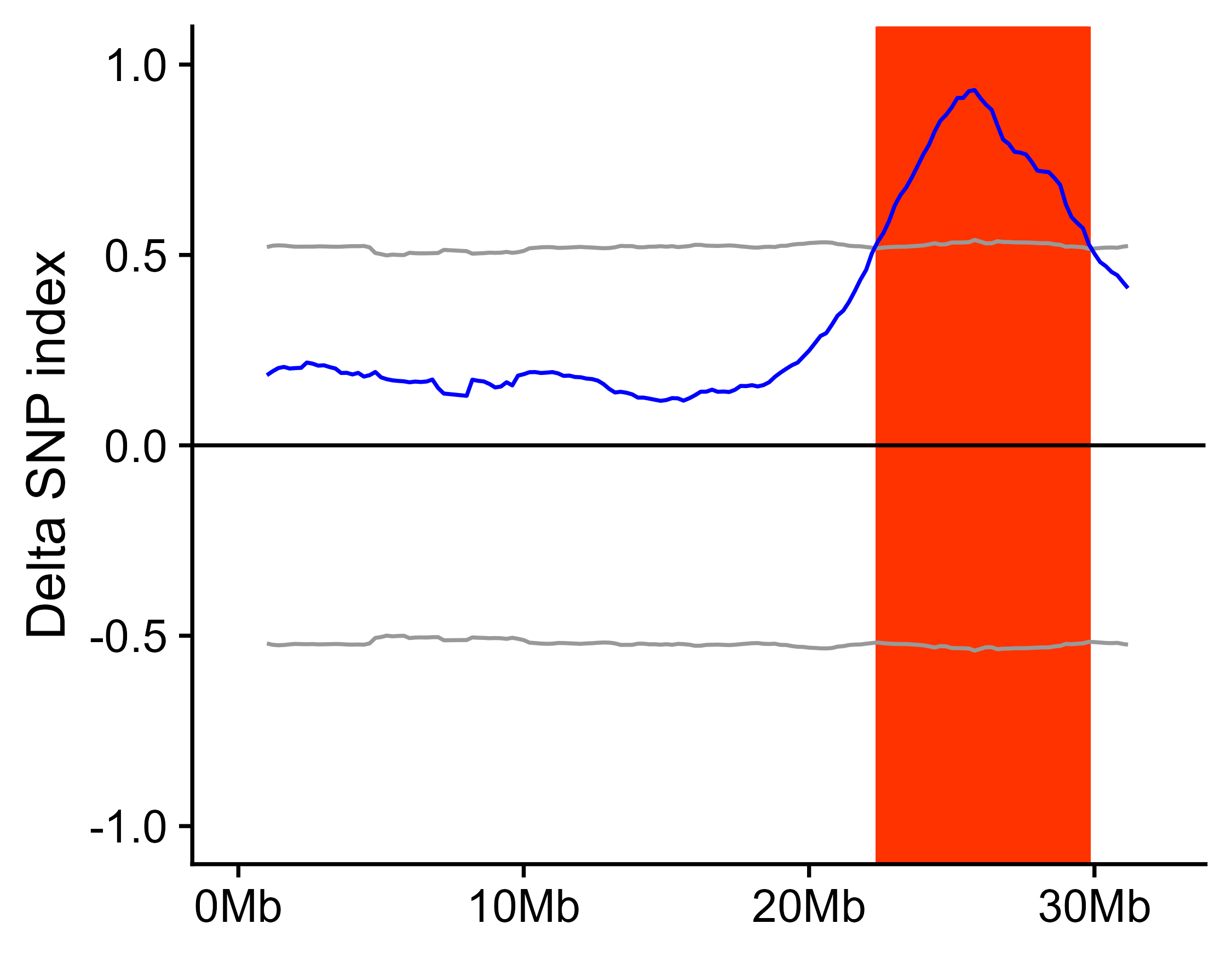
x_filter <- calc_index_etc(x = x_filter, outPrefix = "outprefix", winSize = 2000000, winStep = 200000)After calculating delta SNP index and DE, the result can be show along the chromosome. This function will export the figures.
export_figure(x = x_filter,
outPrefix = "outprefix",
targetChr = c("scaffoldA01", "scaffoldA07", "scaffoldA09"), # Target chromosome to be drawn in figures, default is all chromosomes in the data.
chrLabel = c("A01", "A07", "A09"), # The label for chromosome shown in figures, default is chromosome names in the data.
minN = 20, # Too few SNPs in a window will result in noise, the windows containing SNPs less than minN will be omitted in figures.
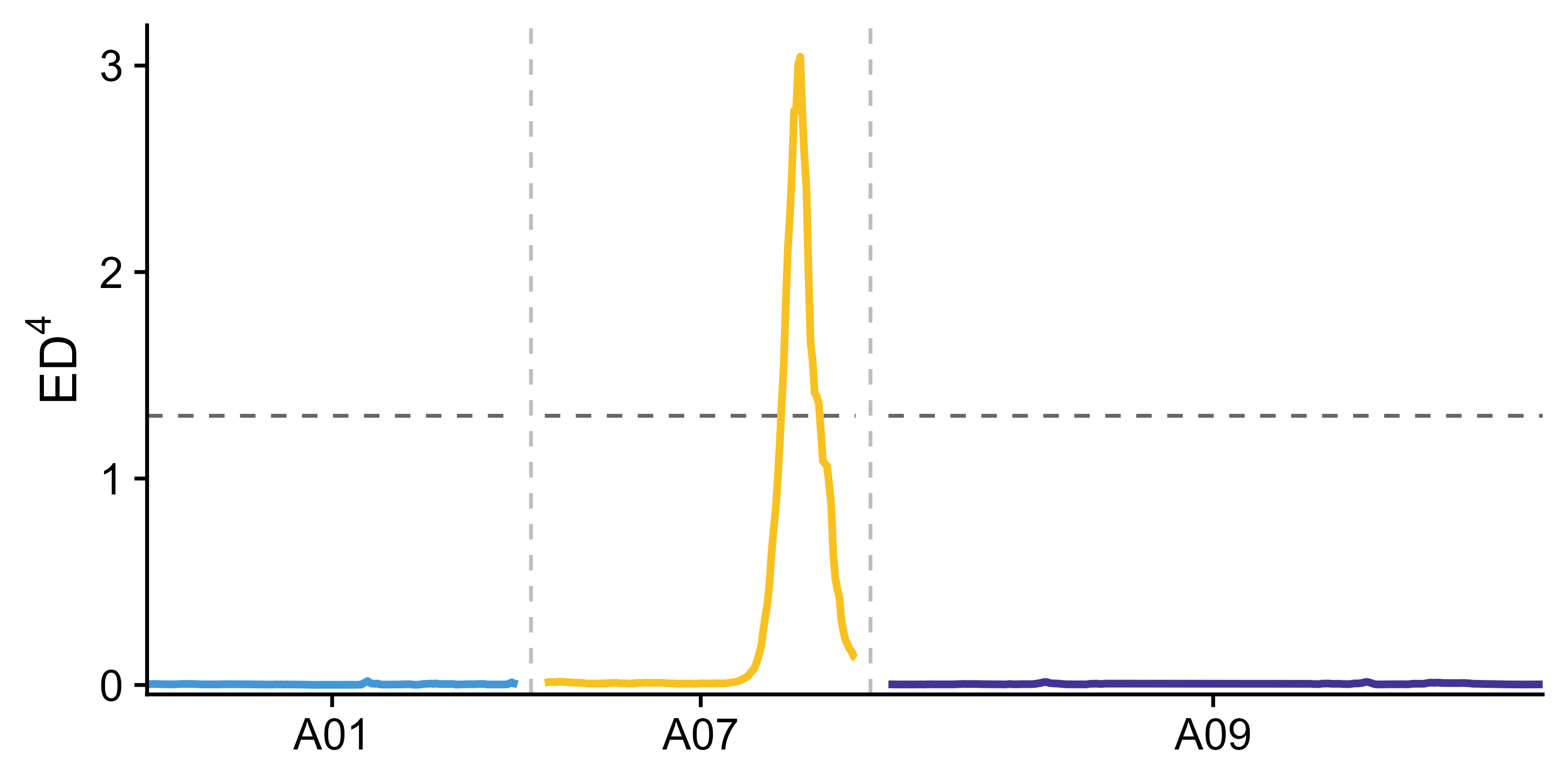
width = 6, height = 3)If parent is present in the data or parent has a reference genome which is used for SNP calling, delta SNP index confidence intervals for different read depths under the null hypothesis (no QTL) as obtained by simulation test (10,000 replications for each read depth). The chromosome regions exceed 95% or 99% confidence intervals are considered as significant QTL region.
getQTL_and_exportFigure(x = x_filter, outPrefix = "outprefix", minN = 20)If this package is used in your research, you should cite this package in the method section, like:
The QTL-seq analysis was performed using R package easyQTLseq (https://github.com/laowang1992/easyQTLseq.git).
This paper is also recommended to cite: