Luis F. Arias-Giraldo, Marlon E. Cobos, A. Townsend Peterson
enmpa is an R package that contains a set of tools to perform
Ecological Niche Modeling using presence-absence data. Some of the main
functions help perform data partitioning, model calibration, model
selection, variable response exploration, and model projection.
If you use enmpa in your research, please cite the following paper:
Arias-Giraldo, Luis F., and Marlon E. Cobos. 2024. “enmpa: An R Package for Ecological Niche Modeling Using Presence-Absence Data and Generalized Linear Models”. Biodiversity Informatics 18. https://doi.org/10.17161/bi.v18i.21742.
To install the stable version of enmpa from CRAN use:
install.packages("enmpa")You can install the development version of enmpa from
GitHub with:
# install.packages("remotes")
remotes::install_github("Luisagi/enmpa")The package terra is used to handle spatial data, and enmpa is used
to perform ENM.
library(enmpa)
library(terra)The data used in this example is included in enmpa.
# Species presence absence data associated with environmental variables
data("enm_data", package = "enmpa")
# Data for final model evaluation
data("test", package = "enmpa")
# Environmental data as raster layers for projections
env_vars <- rast(system.file("extdata", "vars.tif", package = "enmpa"))
# Check the example data
head(enm_data)
#> Sp bio_1 bio_12
#> 1 0 4.222687 403
#> 2 0 6.006802 738
#> 3 0 4.079385 786
#> 4 1 8.418489 453
#> 5 0 8.573750 553
#> 6 1 16.934618 319The raster layers for projections were obtained from WorldClim:
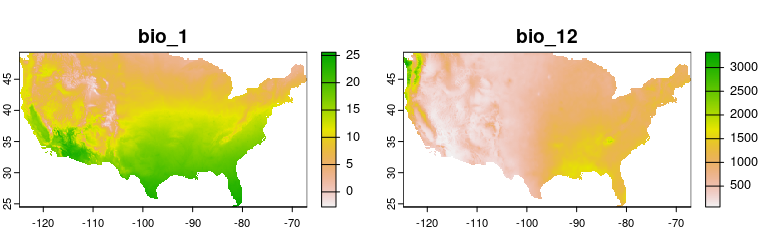
- bio_1 = Annual Mean Temperature
- bio_12 = Annual Precipitation
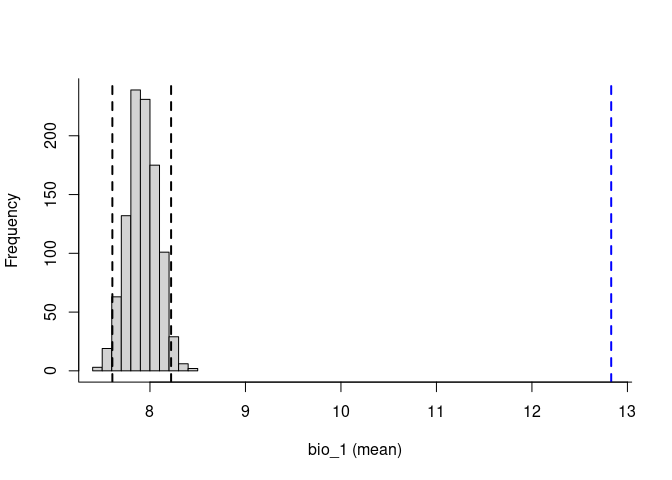
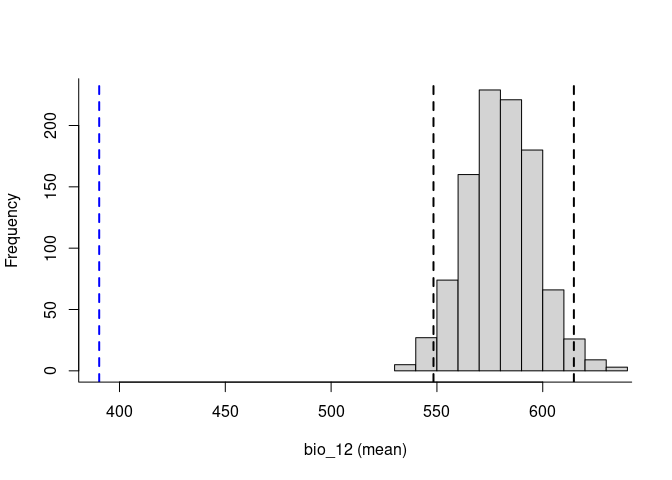
plot(env_vars, mar = c(1, 1, 2, 4))To explore the relevance of the variables to be used in niche models, we implemented in the methods developed by (Cobos and Peterson 2022). These methods help to identify signals of ecological niche considering distinct variables and the presence-absence data. By characterizing the sampling universe, this approach can determine whether presences and absences can be separated better than randomly considering distinct environmental factors.
sn_bio1 <- niche_signal(data = enm_data, variables = "bio_1",
condition = "Sp", method = "univariate")
sn_bio12 <- niche_signal(data = enm_data, variables = "bio_12",
condition = "Sp", method = "univariate")plot_niche_signal(sn_bio1, variables = "bio_1")
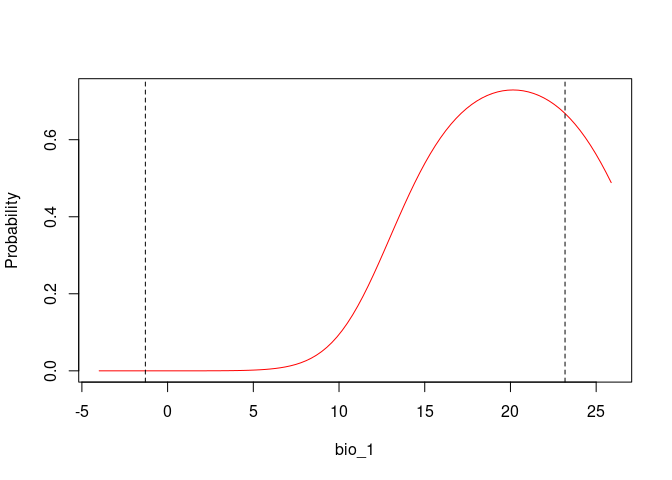
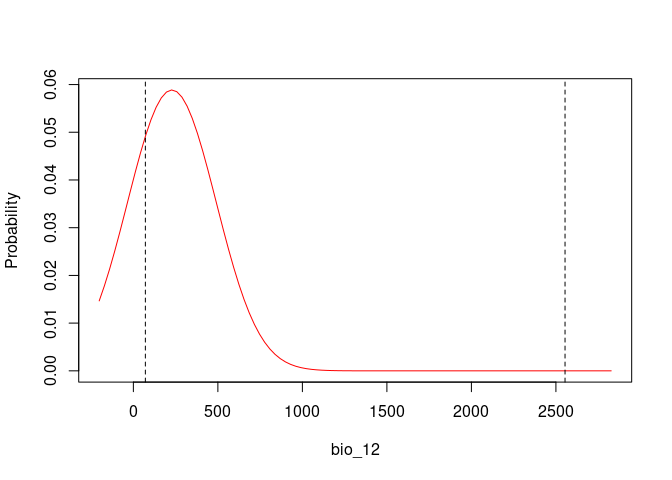
plot_niche_signal(sn_bio12, variables = "bio_12")Based on the univariate test results, the variables bio_1 and bio_12 help to detect signals of ecological niche in our data. In our example, the species tends to occur in areas with higher annual mean temperatures (bio_1); whereas, considering annual precipitation (bio_12), the species seems to be present in areas with lower values. See the function’s documentation for more information.
With enmpa you have the possibility to explore multiple model formulas
derived from combinations of variables considering linear (l), quadratic
(q), and product (p) responses. Product refers to pair interactions of
variables.
The function includes the flag mode to determine what strategy to use
to combine predictors based on the responses defined in . The options of
mode are:
- light.– returns simple iterations of complex formulas.
- moderate.– returns a comprehensive number of iterations.
- intensive.– returns all possible combination. Very time-consuming for 6 or more independent variables.
- complex.– returns only the most complex formula.
An example using linear + quadratic responses:
get_formulas(dependent = "Sp", independent = c("bio_1", "bio_12"),
type = "lq", mode = "intensive")
#> [1] "Sp ~ bio_1"
#> [2] "Sp ~ bio_12"
#> [3] "Sp ~ I(bio_1^2)"
#> [4] "Sp ~ I(bio_12^2)"
#> [5] "Sp ~ bio_1 + bio_12"
#> [6] "Sp ~ bio_1 + I(bio_1^2)"
#> [7] "Sp ~ bio_1 + I(bio_12^2)"
#> [8] "Sp ~ bio_12 + I(bio_1^2)"
#> [9] "Sp ~ bio_12 + I(bio_12^2)"
#> [10] "Sp ~ I(bio_1^2) + I(bio_12^2)"
#> [11] "Sp ~ bio_1 + bio_12 + I(bio_1^2)"
#> [12] "Sp ~ bio_1 + bio_12 + I(bio_12^2)"
#> [13] "Sp ~ bio_1 + I(bio_1^2) + I(bio_12^2)"
#> [14] "Sp ~ bio_12 + I(bio_1^2) + I(bio_12^2)"
#> [15] "Sp ~ bio_1 + bio_12 + I(bio_1^2) + I(bio_12^2)"The function calibration_glm() is a general function that allows to
perform the following steps:
- Partition data in k-folds
- Create formulas for candidate models
- Fit and evaluate candidate models
- Select best performing models
Model selection consists of three steps:
- A first filter to keep the models with ROC AUC >= 0.5 (statistically significant models).
- A second filter to maintain only models that meet a
selection_criterion(“TSS”: TSS >= 0.4; or “ESS”: maximum Accuracy - tolerance). - From those, pick the ones with delta AIC <= 2.
The results are returned as a list containing:
- Selected models (
*$selected) - A summary of statistics for all models (
*$summary) - Results obtained from cross-validation for all models
(
*$calibration_results) - Input data used (
*$data) - The weights used (
*$weights) - The list of partition indices (
*$kfold_index_partition)
Now lets run an example of model calibration and selection:
# Linear + quadratic + products responses
calibration <- calibration_glm(data = enm_data, dependent = "Sp",
independent = c("bio_1", "bio_12"),
response_type = "lpq",
formula_mode = "intensive",
exclude_bimodal = TRUE,
selection_criterion = "TSS",
cv_kfolds = 5, verbose = FALSE)
calibration
#> enmpa-class `enmpa_calibration`:
#> $selected : Selected models (N = 2)
#> $summary : A summary of statistics for all models.
#> $calibration_results : Results obtained from cross-validation for all models.
#> $data : Data used for calibration.
#> $partitioned_data : k-fold indexes (k = 5)
#> $weights : Use of weights (FALSE)Process results:
## Summary of the calibration
summary(calibration)
#>
#> Summary of enmpa_calibration
#> -------------------------------------------------------------------
#>
#> Number of selected models: 2
#> Number of partitions: (k = 5)
#> Weights used: No
#> Summary of selected models (threshold criteria = maxTSS ):
#> ModelID ROC_AUC_mean Accuracy_mean Specificity_mean Sensitivity_mean
#> 1 ModelID_29 0.9003 0.8274 0.8245 0.858
#> 2 ModelID_31 0.9002 0.8305 0.8280 0.856
#> TSS_mean AIC_weight
#> 1 0.6825 0.3751818
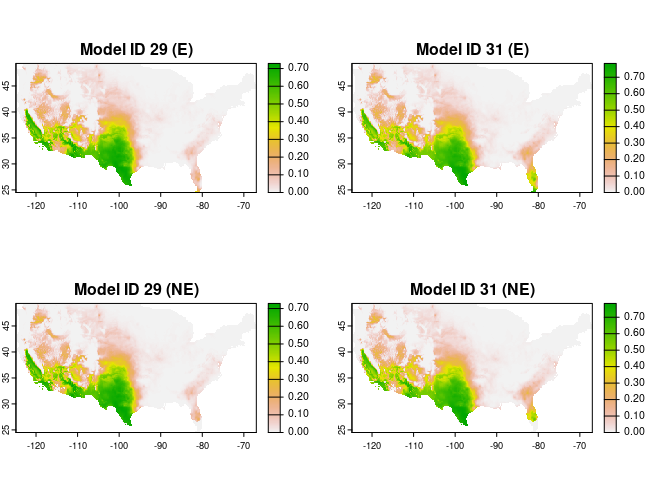
#> 2 0.6840 0.6248182After one or more models are selected, the next steps are the fitting and projection of these models. In this case we are projecting the models to the whole area of interest. Models can be transferred with three options: free extrapolation (‘E’), extrapolation with clamping (‘EC’), and no extrapolation (‘NE’).
# Fitting selected models
fits <- fit_selected(calibration)
# Prediction for the two selected models and their consensus
preds_E <- predict_selected(fits, newdata = env_vars, extrapolation_type = "E",
consensus = TRUE)
preds_NE <- predict_selected(fits, newdata = env_vars,extrapolation_type = "NE",
consensus = TRUE)
# Visualization
plot(c(preds_E$predictions, preds_NE$predictions),
main = c("Model ID 29 (E)", "Model ID 31 (E)",
"Model ID 29 (NE)", "Model ID 31 (NE)"),
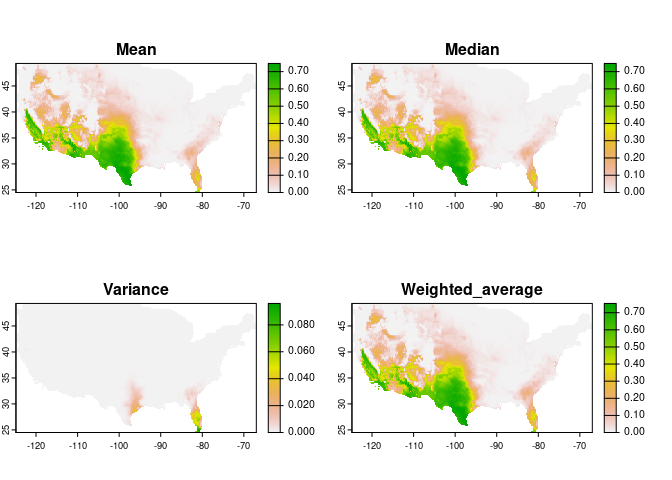
mar = c(1, 1, 2, 5))An alternative to strict selection of a single model is to use a consensus model. The main idea is to avoid selecting the best model and instead rely on multiple models with similar performance.
During the prediction of selected models, we calculated model consensus using the mean, median, and a weighted average (using Akaike weights) (Akaike 1973; Wagenmakers and Farrell 2004).
# Consensus projections
plot(preds_E$consensus, mar = c(1, 1, 2, 5))An important step in understanding the ecological niches with these models is to explore variable response curves. The following lines of code help to do so:
# Response Curves for Bio_1 and Bio_2, first selected model
response_curve(fitted = fits, modelID = "ModelID_29", variable = "bio_1")
response_curve(fitted = fits, modelID = "ModelID_29", variable = "bio_12")# Consensus Response Curves for Bio_1 and Bio_2, for both models
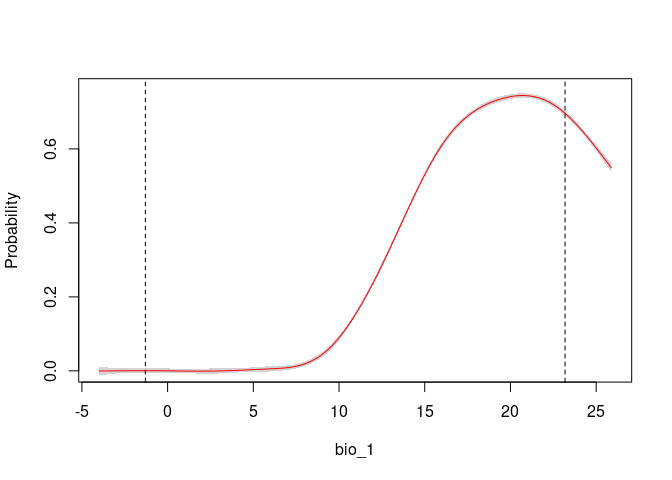
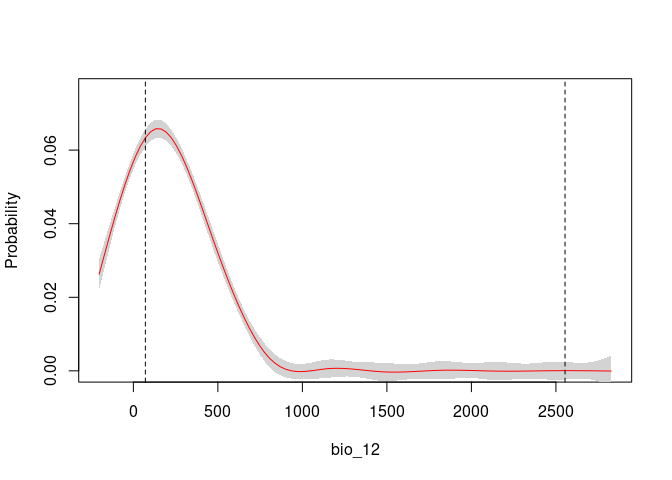
response_curve(fits, variable = "bio_1")
response_curve(fits, variable = "bio_12")It is useful to examine whether the effect of one variable depends on
the level of other variables. If it does, then we have what is called an
‘interaction’. According to the calibration results from this example,
in both models, the predictor bio_1:bio_12 was selected. To explore
the interaction of these two variables, the function resp2var can help
us to visualize this interaction.
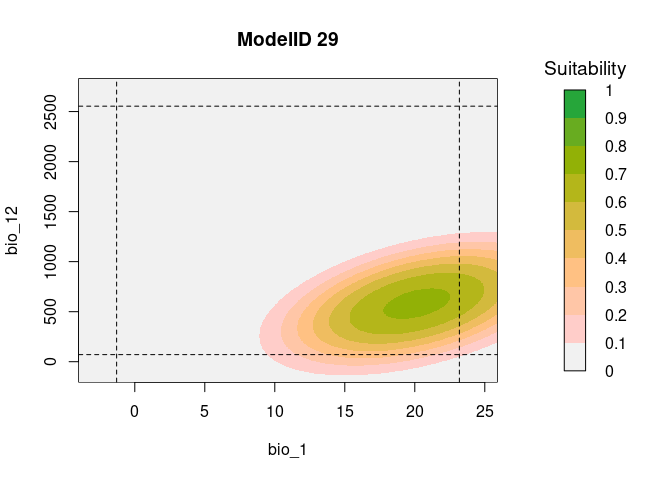
# Consensus Response Curves for Bio_1 and Bio_2, for both models
resp2var(model = fits,
modelID = "ModelID_29",
main = "ModelID 29",
variable1 = "bio_1",
variable2 = "bio_12",
extrapolate = TRUE,
add_limits = TRUE)
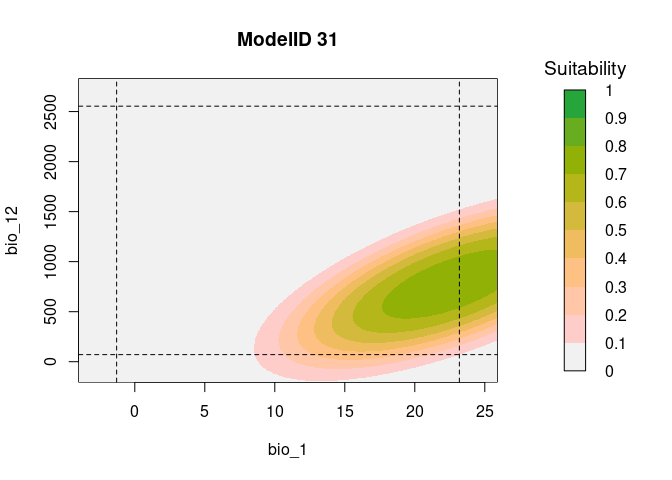
resp2var(model = fits,
modelID = "ModelID_31",
main = "ModelID 31",
variable1 = "bio_1",
variable2 = "bio_12",
extrapolate = TRUE,
add_limits = TRUE)Variable importance or contribution to models can be calculated as a function of the relative deviance explained by each predictor.
Analysis of Deviance for the first selected model:
anova(fits$glms_fitted$ModelID_29, test = "Chi")
#> Analysis of Deviance Table
#>
#> Model: binomial, link: logit
#>
#> Response: Sp
#>
#> Terms added sequentially (first to last)
#>
#>
#> Df Deviance Resid. Df Resid. Dev Pr(>Chi)
#> NULL 5626 3374.9
#> bio_1 1 869.35 5625 2505.6 < 2.2e-16 ***
#> I(bio_1^2) 1 46.77 5624 2458.8 7.972e-12 ***
#> I(bio_12^2) 1 239.69 5623 2219.1 < 2.2e-16 ***
#> bio_1:bio_12 1 42.40 5622 2176.7 7.450e-11 ***
#> ---
#> Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1Using a function from enmpa you can also explore variable importance
in terms of contribution.
# Relative contribution of the deviance explained for the first model
var_importance(fitted = fits, modelID = "ModelID_29")
#> predictor contribution cum_contribution
#> 3 I(bio_12^2) 0.3572673 0.3572673
#> 1 bio_1 0.2919587 0.6492259
#> 2 I(bio_1^2) 0.2031750 0.8524009
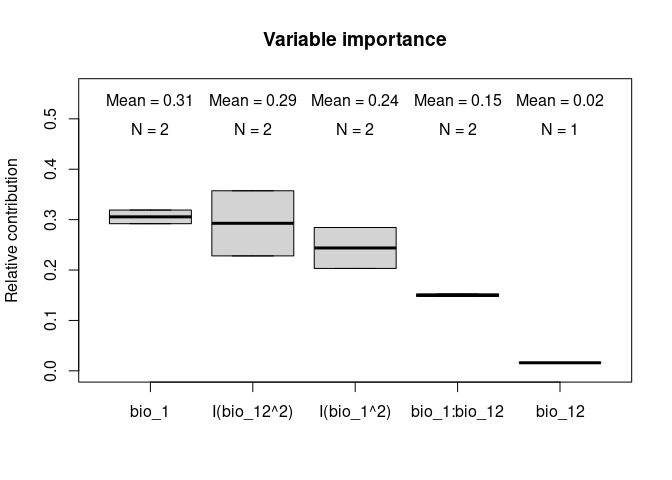
#> 4 bio_1:bio_12 0.1475991 1.0000000The function also allows to plot the contributions of the variables for the two models together which can help with the interpretations:
# Relative contribution of the deviance explained
vi_both_models <- var_importance(fits)# Plot
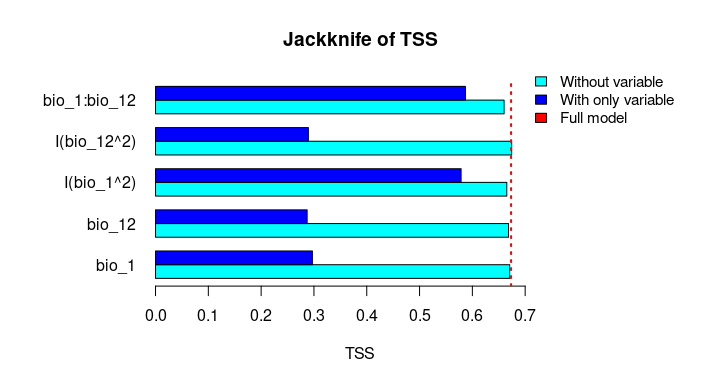
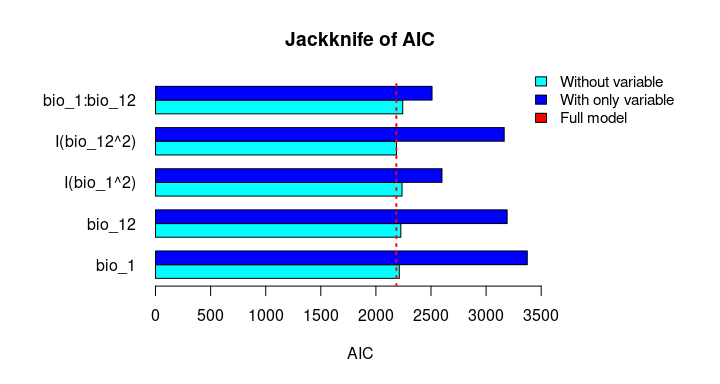
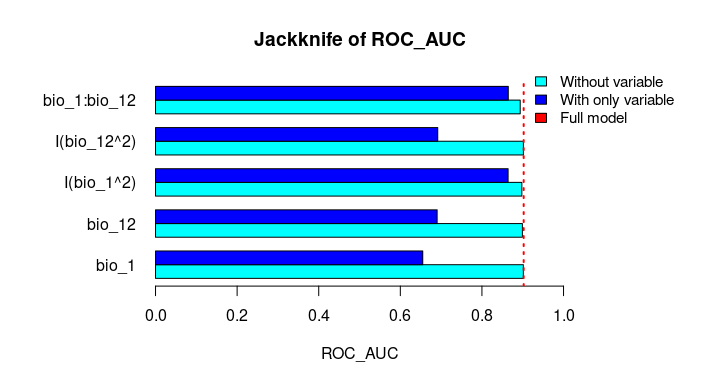
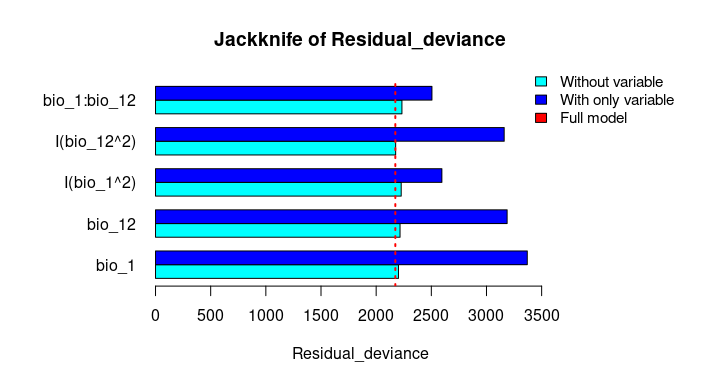
plot_importance(vi_both_models, extra_info = TRUE)The Jackknife function providing a detailed reflection of the impact of each variable on the overall model, considering four difference measures: ROC-AUC, TSS, AICc, and Deviance.
# Jackknife test
jk <- jackknife(data = enm_data,
dependent = "Sp",
independent = c("bio_1", "bio_12"),
response_type = "lpq")
jk
#> $Full_model_stats
#> ROC_AUC TSS AIC Residual_deviance
#> 1 0.9023279 0.6732916 2185.68 2173.68
#>
#> $Formula
#> [1] "Sp ~ bio_1 + bio_12 + I(bio_1^2) + I(bio_12^2) + bio_1:bio_12"
#>
#> $Without
#> ROC_AUC TSS AIC Residual_deviance
#> bio_1 0.9015319 0.6707435 2212.307 2202.307
#> bio_12 0.8991734 0.6684712 2226.488 2216.488
#> I(bio_1^2) 0.8978504 0.6651925 2237.051 2227.051
#> I(bio_12^2) 0.9016647 0.6744525 2186.700 2176.700
#> bio_1:bio_12 0.8938496 0.6600718 2243.562 2233.562
#>
#> $With_only
#> ROC_AUC TSS AIC Residual_deviance
#> bio_1 0.6549700 0.2970911 3373.978 3369.978
#> bio_12 0.6902666 0.2871473 3191.205 3187.205
#> I(bio_1^2) 0.8641785 0.5786819 2599.638 2595.638
#> I(bio_12^2) 0.6916871 0.2894878 3164.568 3160.568
#> bio_1:bio_12 0.8646620 0.5868336 2509.565 2505.565# Jackknife plots
plot_jk(jk, metric = "TSS")
plot_jk(jk, metric = "AIC")
plot_jk(jk, metric = "ROC_AUC")
plot_jk(jk, metric = "Residual_deviance")Finally, we will evaluate the final models using the “independent_eval” functions. Ideally, the model should be evaluated with an independent data set (i.e., data that was not used during model calibration or for final model fitting).
# Loading an independent dataset
data("test", package = "enmpa")
# The independent evaluation data are divided into two groups:
# presences-absences (test_01) and presences-only (test_1).
test_1 <- test[test$Sp == 1,]
test_01 <- test
head(test_1)
#> Sp lon lat
#> 4 1 -117.5543 33.62975
#> 8 1 -100.9363 38.68424
#> 19 1 -110.4748 37.78367
#> 53 1 -106.2348 31.70118
#> 54 1 -112.3623 39.35173
#> 60 1 -109.3399 38.26670
head(test_01)
#> Sp lon lat
#> 1 0 -105.6639 35.81247
#> 2 0 -107.9354 33.37200
#> 3 0 -100.3134 48.96018
#> 4 1 -117.5543 33.62975
#> 5 0 -120.6168 36.59670
#> 6 0 -105.3379 40.08928Using independent data for which presence and absence is known can give the most robust results. Here an example:
projections <- list(
ModelID_29 = preds_E$predictions$ModelID_29,
ModelID_31 = preds_E$predictions$ModelID_31,
Consensus_WA = preds_E$consensus$Weighted_average
)
ie_01 <- lapply(projections, function(x){
independent_eval01(prediction = x,
observation = test_01$Sp,
lon_lat = test_01[, c("lon", "lat")])
})
ie_01
#> $ModelID_29
#> Threshold_criteria Threshold ROC_AUC False_positive_rate Accuracy
#> 204 ESS 0.1365298 0.9570991 0.08988764 0.91
#> 175 maxTSS 0.1170256 0.9570991 0.10112360 0.91
#> 196 SEN90 0.1311493 0.9570991 0.10112360 0.90
#> Sensitivity Specificity TSS
#> 204 0.9090909 0.9101124 0.8192033
#> 175 1.0000000 0.8988764 0.8988764
#> 196 0.9090909 0.8988764 0.8079673
#>
#> $ModelID_31
#> Threshold_criteria Threshold ROC_AUC False_positive_rate Accuracy
#> 239 ESS 0.1579818 0.9570991 0.08988764 0.91
#> 205 maxTSS 0.1354130 0.9570991 0.10112360 0.91
#> 218 SEN90 0.1440422 0.9570991 0.10112360 0.90
#> Sensitivity Specificity TSS
#> 239 0.9090909 0.9101124 0.8192033
#> 205 1.0000000 0.8988764 0.8988764
#> 218 0.9090909 0.8988764 0.8079673
#>
#> $Consensus_WA
#> Threshold_criteria Threshold ROC_AUC False_positive_rate Accuracy
#> 226 ESS 0.1500930 0.9570991 0.08988764 0.91
#> 192 maxTSS 0.1274123 0.9570991 0.10112360 0.91
#> 210 SEN90 0.1394197 0.9570991 0.10112360 0.90
#> Sensitivity Specificity TSS
#> 226 0.9090909 0.9101124 0.8192033
#> 192 1.0000000 0.8988764 0.8988764
#> 210 0.9090909 0.8988764 0.8079673When only presence data is available, the evaluation of performance is based on the omission error and the partial ROC analysis.
To do this in our example, we will need to define a threshold value. We can use any of the three threshold values obtained above: ESS, maxTSS or SEN90.
# In this example, we will use the weighted average of the two selected models.
# Consensus_WA
Consensus_WA_T1 <- ie_01[["Consensus_WA"]][1, "Threshold"] # ESS
Consensus_WA_T2 <- ie_01[["Consensus_WA"]][2, "Threshold"] # maxTSS
Consensus_WA_T3 <- ie_01[["Consensus_WA"]][3, "Threshold"] # SEN90
aux_list <- list(ESS = Consensus_WA_T1, maxTSS = Consensus_WA_T2,
SEN90 = Consensus_WA_T3)
lapply(aux_list, function(th){
independent_eval1(prediction = projections[["Consensus_WA"]],
threshold = th,
lon_lat = test_1[, c("lon", "lat")])
})
#> $ESS
#> omission_error threshold Mean_AUC_ratio pval_pROC
#> 1 0.09090909 0.150093 1.634595 0
#>
#> $maxTSS
#> omission_error threshold Mean_AUC_ratio pval_pROC
#> 1 0 0.1274123 1.63588 0
#>
#> $SEN90
#> omission_error threshold Mean_AUC_ratio pval_pROC
#> 1 0.09090909 0.1394197 1.632866 0Akaike, H. 1973. “Information Theory and an Extension of Maximum Likelihood Principle.” In Proc. 2nd Int. Symp. On Information Theory, 267–81.
Cobos, Marlon E., and A. Townsend Peterson. 2022. “Detecting Signals of Species’ Ecological Niches in Results of Studies with Defined Sampling Protocols: Example Application to Pathogen Niches.” Biodiversity Informatics 17 (May): 50–58. https://doi.org/10.17161/bi.v17i.15985.
Wagenmakers, Eric-Jan, and Simon Farrell. 2004. “AIC Model Selection Using Akaike Weights.” Psychonomic Bulletin & Review 11 (1): 192–96. https://doi.org/10.3758/BF03206482.