IDRPred is a modern implementation of MobiDB-lite[1], a method for identifying intrinsically disordered regions (IDRs) in proteins. MobiDB-lite uses multiple predictors to derive a consensus, which is filtered for spurious short predictions in a second step.
The main advantage of IDRPred is that it only requires Python 3 while MobiDB-lite requires both Python 2 and 3.
pip install git+https://github.com/matthiasblum/idrpredA Docker image of idrpred is available from Docker Hub.
idrpred [options] [infile] [outfile]Positional arguments:
infile: The FASTA file of sequences to process. If-or not specified, read from standard input.outfile: The TSV file of predicted intrinsically disordered regions. If-or not specified, write to standard output.
| Options | Description |
|---|---|
--force |
Derive a consensus as long as one predictor did not fail |
--skip-features |
Do not indentify sequence features, such as domains of low complexity |
--round |
Round scores reported by individual predictors, like MobiDB-lite does |
--tempdir PATH |
Create temporary files in PATH, instead of the default temporary directory (most likely /tmp) |
--threads N |
Process up to N sequences concurrently, default: 1 |
Only predictors whose licence authorises distribution have been included in IDRPred.
| Method | Reference | Available |
|---|---|---|
| ANCHOR | [2] | ❌ |
| DisEMBL-465 | [3] | ✔ |
| DisEMBL-HotLoops | [3] | ✔ |
| DynaMine | [4] | ❌ |
| ESpritz-DisProt | [5] | ✔ |
| ESpritz-NMR | [5] | ✔ |
| ESpritz-Xray | [5] | ✔ |
| FeSS | [6] | ❌ |
| GlobPlot | [7] | ✔ |
| IUPred-Long | [8] | ✔ |
| IUPred-Short | [8] | ✔ |
| JRONN | [9] | ❌ |
| Pfilt | [10] | ❌ |
| SEG | [11] | ✔ |
| VSL2b | [12] | ❌ |
| Reference proteome | Sequences | Default options | IDRPred: --round option |
|---|---|---|---|
| A. thaliana | 39,320 |  |
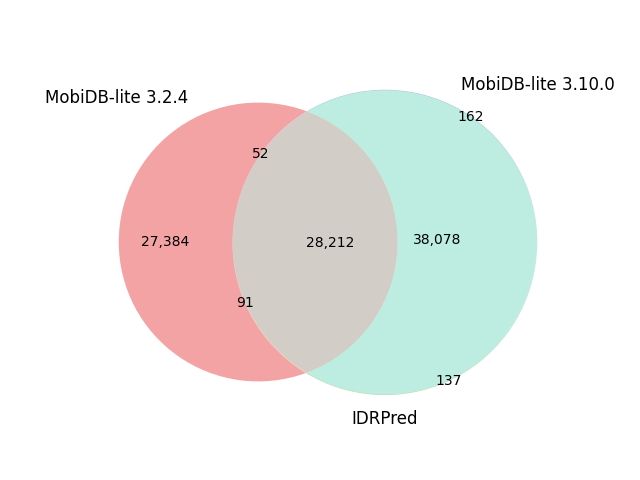 |
| D. melanogaster | 26,706 | 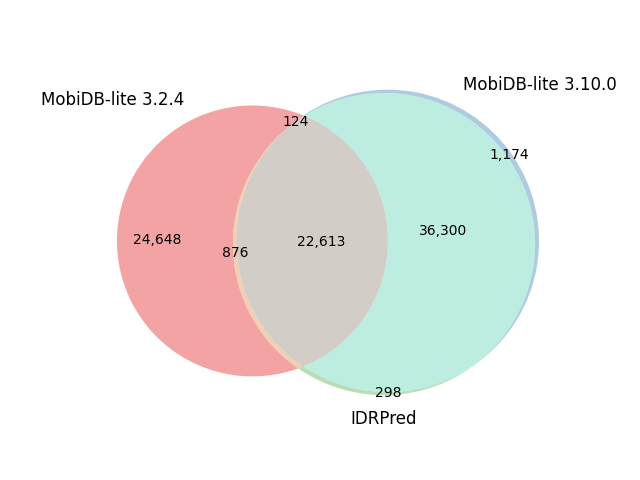 |
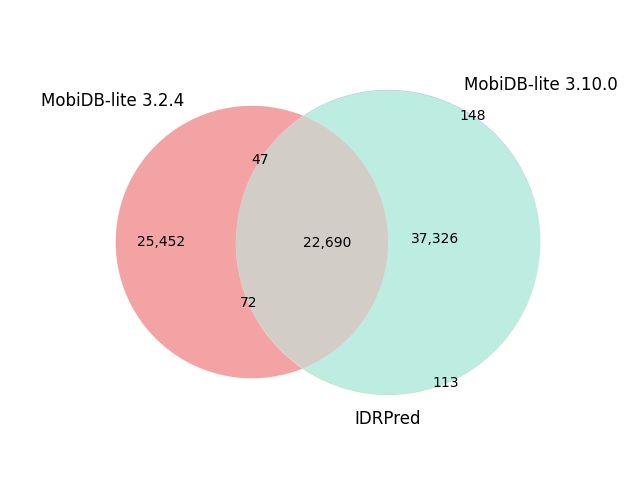 |
| E. Coli | 4,403 | 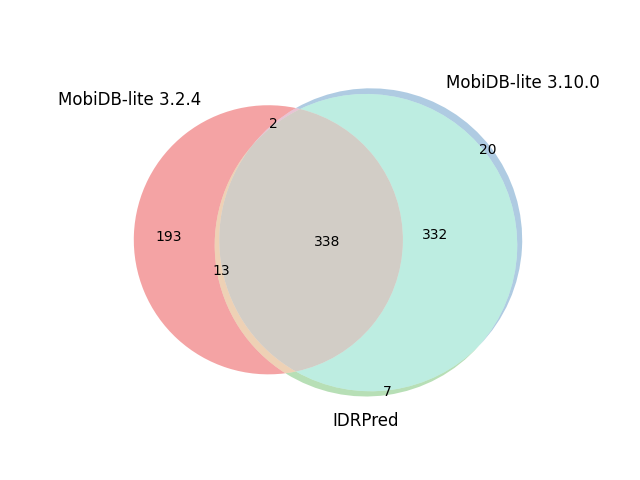 |
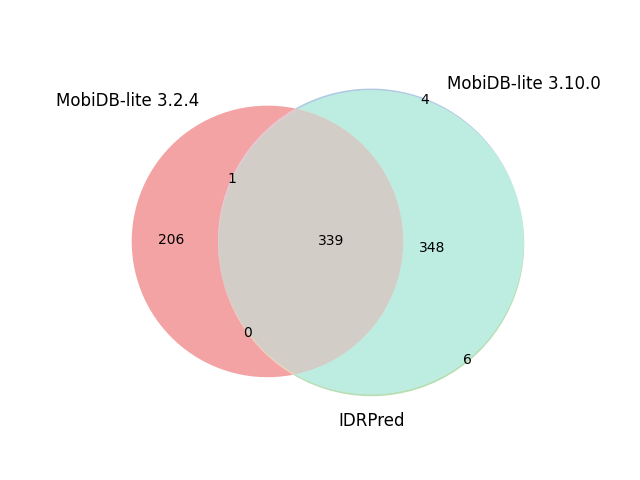 |
| H. Sapiens | 82,492 | 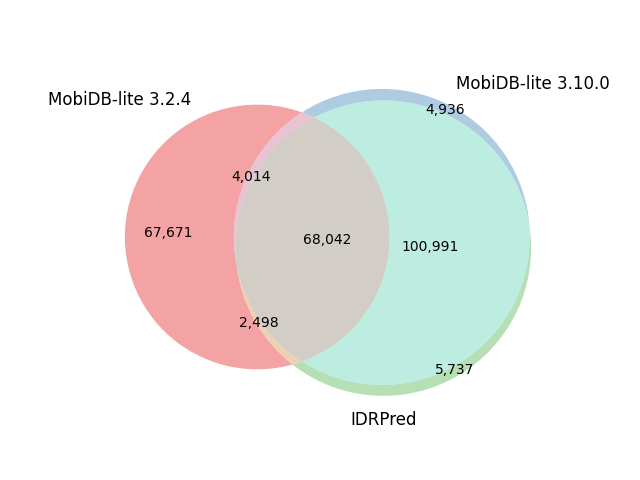 |
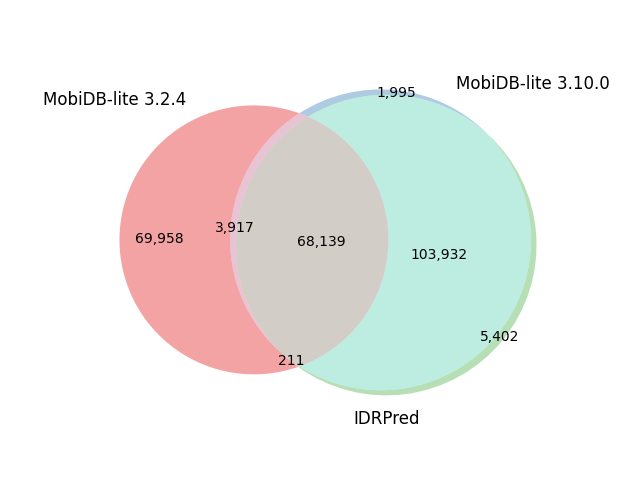 |
| S. cerevisiae | 6,060 | 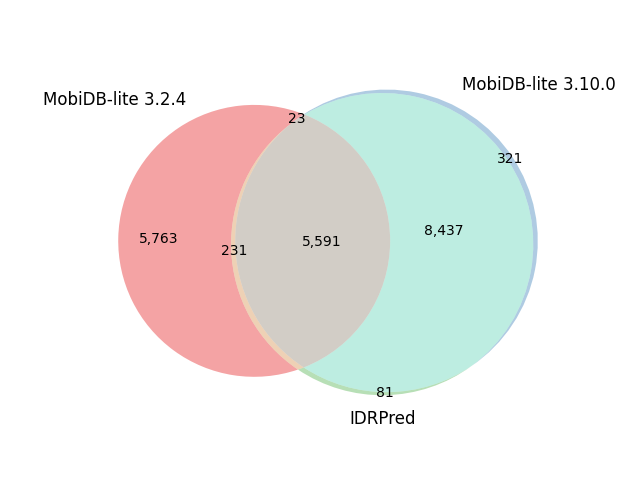 |
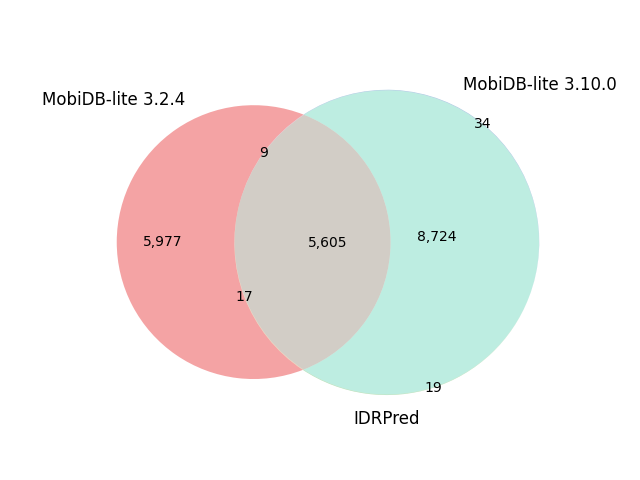 |
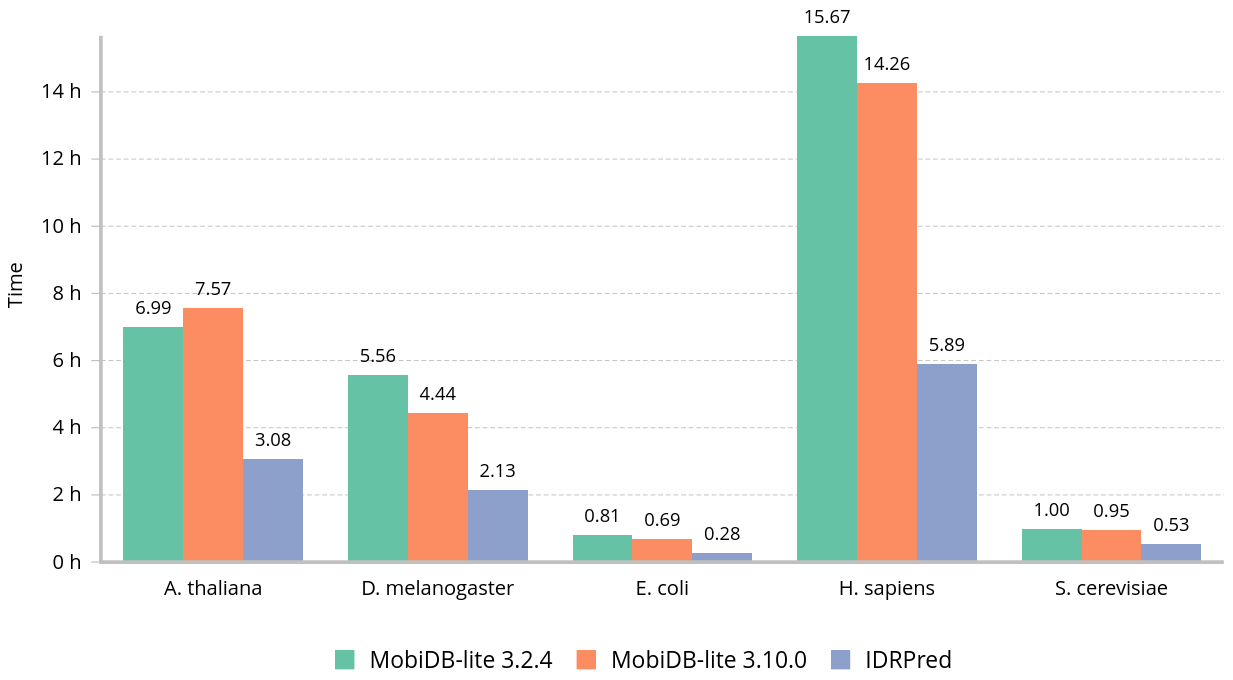
Wall clock time to annotate common proteomes using one thread:
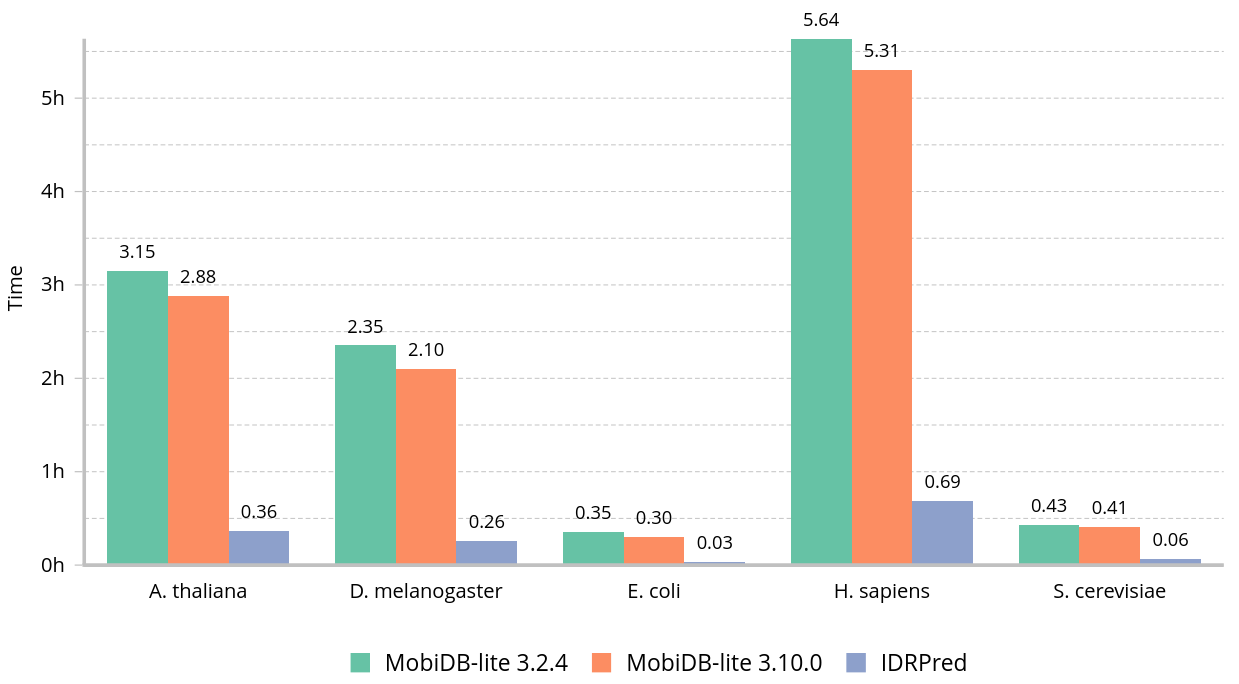
Wall clock time to annotate common proteomes using eight threads:
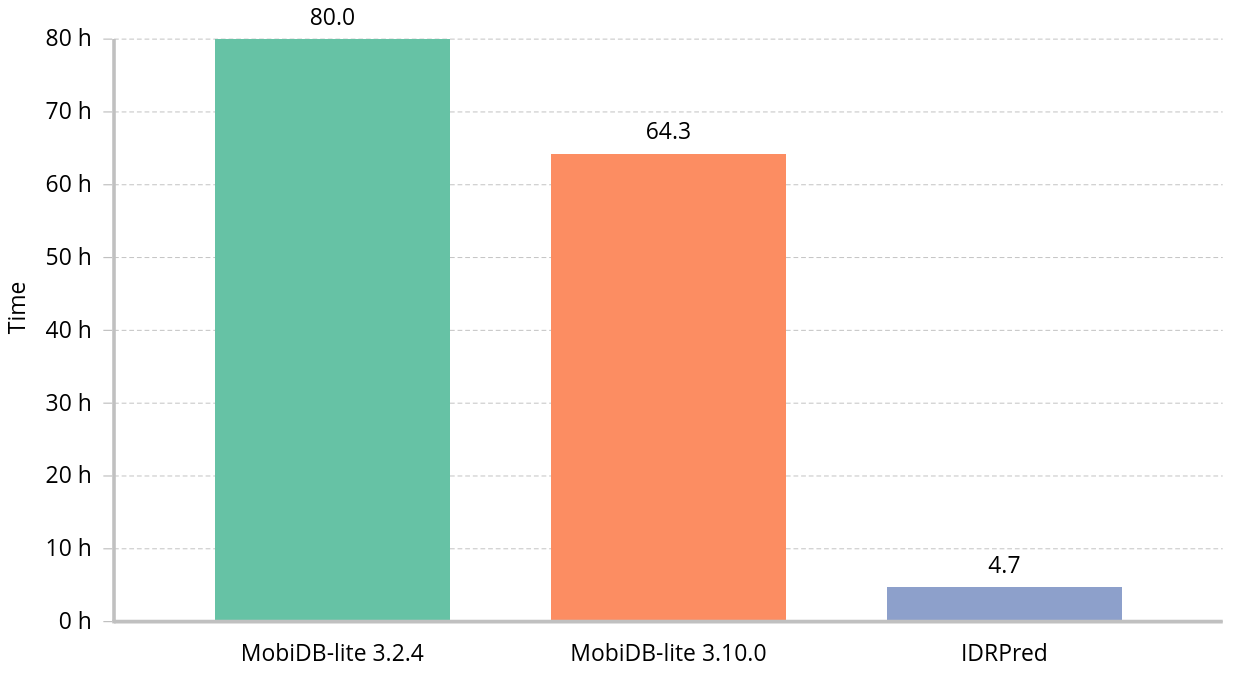
Wall clock time to annotate one million sequences randomly selected from UniParc using sixteen threads:
- Necci M, Piovesan D, Clementel D, Dosztányi Z, Tosatto SCE. MobiDB-lite 3.0: fast consensus annotation of intrinsic disorder flavors in proteins. Bioinformatics. 2021 Apr 1;36(22-23):5533-5534. DOI: 10.1093/bioinformatics/btaa1045. PMID: 33325498.
- Dosztányi Z, Mészáros B, Simon I. ANCHOR: web server for predicting protein binding regions in disordered proteins. Bioinformatics. 2009 Oct 15;25(20):2745-6. DOI: 10.1093/bioinformatics/btp518. Epub 2009 Aug 28. PMID: 19717576; PMCID: PMC2759549.
- Linding R, Jensen LJ, Diella F, Bork P, Gibson TJ, Russell RB. Protein disorder prediction: implications for structural proteomics. Structure. 2003 Nov;11(11):1453-9. DOI: 10.1016/j.str.2003.10.002. PMID: 14604535.
- Cilia E, Pancsa R, Tompa P, Lenaerts T, Vranken WF. From protein sequence to dynamics and disorder with DynaMine. Nat Commun. 2013;4:2741. DOI: 10.1038/ncomms3741. PMID: 24225580.
- Walsh I, Martin AJ, Di Domenico T, Tosatto SC. ESpritz: accurate and fast prediction of protein disorder. Bioinformatics. 2012 Feb 15;28(4):503-9. DOI: 10.1093/bioinformatics/btr682. Epub 2011 Dec 20. PMID: 22190692.
- Piovesan D, Walsh I, Minervini G, Tosatto SCE. FELLS: fast estimator of latent local structure. Bioinformatics. 2017 Jun 15;33(12):1889-1891. DOI: 10.1093/bioinformatics/btx085. PMID: 28186245.
- Linding R, Russell RB, Neduva V, Gibson TJ. GlobPlot: Exploring protein sequences for globularity and disorder. Nucleic Acids Res. 2003 Jul 1;31(13):3701-8. DOI: 10.1093/nar/gkg519. PMID: 12824398; PMCID: PMC169197.
- Mészáros B, Erdos G, Dosztányi Z. IUPred2A: context-dependent prediction of protein disorder as a function of redox state and protein binding. Nucleic Acids Res. 2018 Jul 2;46(W1):W329-W337. DOI: 10.1093/nar/gky384. PMID: 29860432; PMCID: PMC6030935.
- Yang ZR, Thomson R, McNeil P, Esnouf RM. RONN: the bio-basis function neural network technique applied to the detection of natively disordered regions in proteins. Bioinformatics. 2005 Aug 15;21(16):3369-76. DOI: 10.1093/bioinformatics/bti534. Epub 2005 Jun 9. PMID: 15947016.
- Jones DT, Swindells MB. Getting the most from PSI-BLAST. Trends Biochem Sci. 2002 Mar;27(3):161-4. DOI: 10.1016/s0968-0004(01)02039-4. PMID: 11893514.
- Wootton JC. Non-globular domains in protein sequences: automated segmentation using complexity measures. Comput Chem. 1994 Sep;18(3):269-85. DOI: 10.1016/0097-8485(94)85023-2. PMID: 7952898.
- Peng K, Radivojac P, Vucetic S, Dunker AK, Obradovic Z. Length-dependent prediction of protein intrinsic disorder. BMC Bioinformatics. 2006 Apr 17;7:208. DOI: 10.1186/1471-2105-7-208. PMID: 16618368; PMCID: PMC1479845.