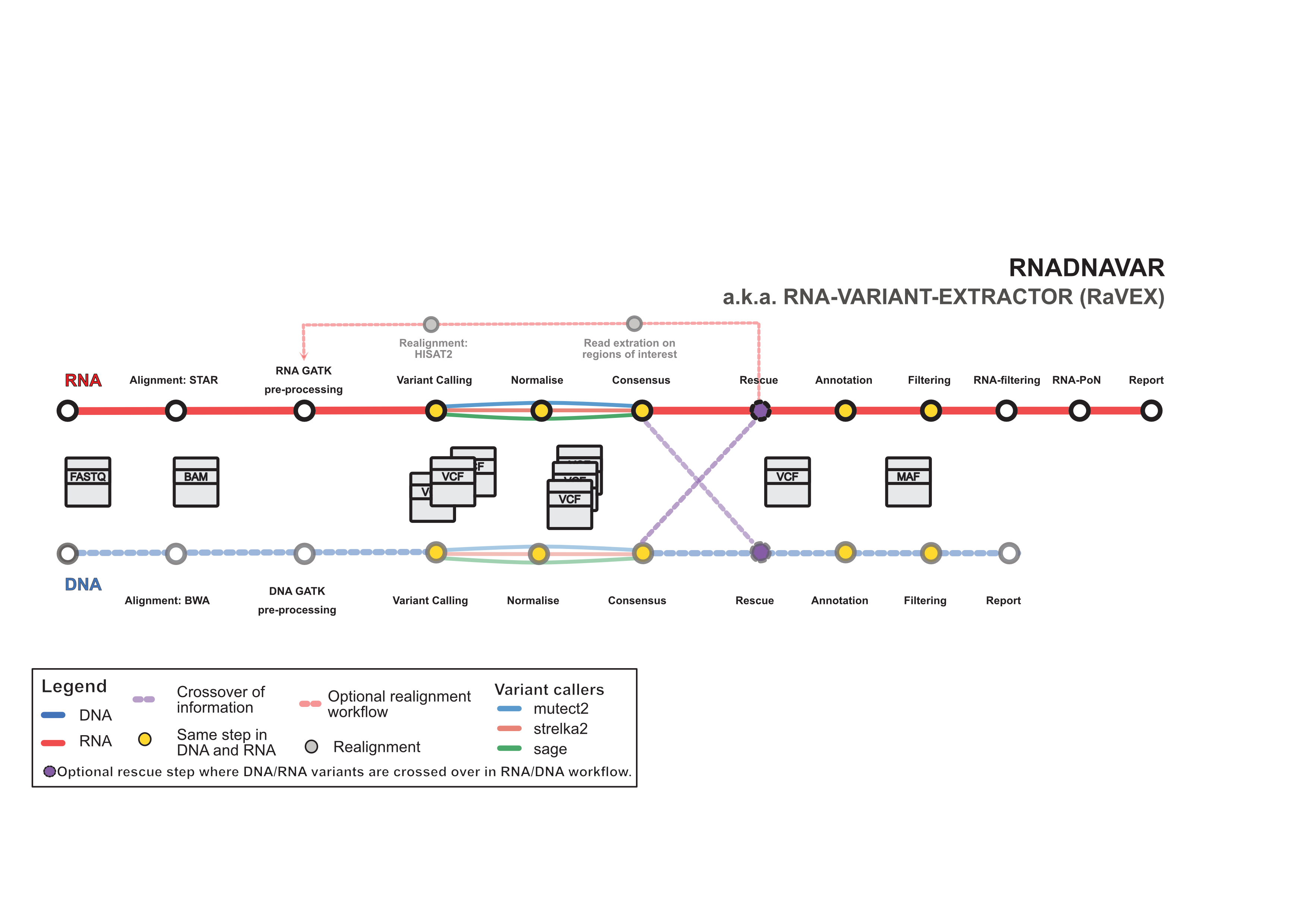
The nf-core/rnadnavar is a bioinformatics best-practice analysis pipeline for RNA somatic mutation detection able to perform in parallel.
Initially designed for cancer research, the pipeline uses different variant calling algorithms and applies a consensus approach. A final filtering stage, should provide a set of annotated somatic variants.
The pipeline is built using Nextflow, a workflow tool to run tasks across multiple compute infrastructures in a very portable manner. It uses Docker/Singularity containers making installation trivial and results highly reproducible. The Nextflow DSL2 implementation of this pipeline uses one container per process which makes it much easier to maintain and update software dependencies. Where possible, these processes have been submitted to and installed from nf-core/modules in order to make them available to all nf-core pipelines, and to everyone within the Nextflow community!
Depending on the options and samples provided, the
pipeline will run different tasks. This is controlled
mainly through --step and --tools parameters. This
is a summary of the possible tasks to run with the pipeline:
- Quality control and trimming (enabled by
--trim_fastqand runsFastQCandfastp) - Map Reads to Reference (BWA-mem, BWA-mem2, dragmap and/or STAR)
- GATK preprocessing for DNA and RNA bulk sequencing
samples (
GATK MarkDuplicates,GATK SplitNCigarReads,GATK BaseRecalibratorandGATK ApplyBQSR) - Summarise alignment statistics (
samtools stats,mosdepth) - Variant calling (enabled with
--tools)Mutect2Strelka2SAGE
- Annotation with
VEP(enabled with--toolsaddingvep) - Normalisation of VCFs with VT (enabled with
--toolsaddingnormalisation) - Transformation of VCF to MAF and consensus of variant
calling results (enabled with
--toolsaddingconsensus) - Filtering of MAF files applying optional gnomad,
whitelisting and blacklisting (enabled with
--toolsaddingfiltering) - Realignment step where reads from regions where a variant
was found will be extracted and re-processed, only for
RNA due to higher levels of background noise (enabled
with
--toolsaddingrealignment). - Filtering of MAF files specific for RNA (enabled with
--toolsaddingrna_filtering)
Note
If you are new to Nextflow and nf-core, please refer to this page on how to set-up Nextflow. Make sure to test your setup with -profile test before running the workflow on actual data.
Now, you can run the pipeline using:
nextflow run nf-core/rnadnavar \
-profile <docker/singularity/.../institute> \
--input samplesheet.csv \
--outdir <OUTDIR>Warning
Please provide pipeline parameters via the CLI or Nextflow -params-file option. Custom config files including those provided by the -c Nextflow option can be used to provide any configuration except for parameters;
see docs.
For more details and further functionality, please refer to the usage documentation and the parameter documentation.
To see the results of an example test run with a full size dataset refer to the results tab on the nf-core website pipeline page. For more details about the output files and reports, please refer to the output documentation.
The nf-core/rnadnavar was originally written by Raquel Manzano Garcia at Cancer Research UK Cambridge Institute with the continuous support of Maxime U Garcia. The workflow is based on RNA-MuTect which was originally published by Yizhak, et al 2019 (Science)
We thank the following people for their assistance in the development of this pipeline: TBC
If you would like to contribute to this pipeline, please see the contributing guidelines.
For further information or help, don't hesitate to get in touch on the Slack #rnadnavar channel (you can join with this invite).
An extensive list of references for the tools used by the pipeline can be found in the CITATIONS.md file.
You can cite the nf-core publication as follows:
The nf-core framework for community-curated bioinformatics pipelines.
Philip Ewels, Alexander Peltzer, Sven Fillinger, Harshil Patel, Johannes Alneberg, Andreas Wilm, Maxime Ulysse Garcia, Paolo Di Tommaso & Sven Nahnsen.
Nat Biotechnol. 2020 Feb 13. doi: 10.1038/s41587-020-0439-x.