Authors: Sean Maden, Reid Thompson, Kasper Hansen, Abhi Nellore
Set up and maintain an instance, or synchronization, of public DNA methylation (DNAm) arrays from the
Gene Expression Omnibus (GEO). The recountmethylation_instance
resource provides a snakemake workflow including all steps to create HDF5 .h5 and HDF5-SummarizedExperiment
h5se database files of compiled and harmonized DNAm array assays and sample metadata. Expedited setup of a
synchronization environment is supported using sh or conda (Tutorial, below).
The recountmethylation_instance resource can handle data compilations comprising upwards of tens of
thousands of samples and hundreds of studies. If you are instead obtaining data from just one or a few
samples or studies from GEO, there are several more lightweight approaches available to you, including
manual download of files from the GEO website, and programmatic options including functions provided in the GEOquery library, the getGenomicRatioSetFromGEO()
function from the minfi library, and
the gds_idatquery() and gds_idat2rg() functions from the recountmethylation library.
Dependencies to run an instance are shown in the below tables showing dependency name (column 1), type (column 2), confirmed version (column 3), and requirement (column 4). Instances were confirmed to work with the indicated versions (column 2), and in many cases can work with more recent versions. Several dependencies are optional to run specific features of an instance, as indicated in the "Required?" column.
Main software and programming language dependencies:
| Dependency | Type | Version | Required? |
|---|---|---|---|
| Python 3 | programming language | >=3.7.3 | yes |
| R | programming language | >=3.6.0 | yes |
| RabbitMQ | broker | 3.8.11 | yes |
| MongoDB | db syntax | 4.4.3 | yes |
| SQLite | db syntax | 3.30.1 | yes |
| Entrez Utilities | NCBI/GEO API utils | 10.9 | yes |
| Python 2 | programming language | 2.7.5 | no |
The following Python 3 libraries are required or recommended. Note packages
dash and plotly are only required to run the optional server dashboard utility:
| Library | Type | Version | Required? |
|---|---|---|---|
| snakemake | Python 3 library | 6.1.2 | yes |
| pandas | Python 3 library | 0.25.1 | yes |
| celery | Python 3 library | 4.2.1 | yes |
| pymongo | Python 3 library | 3.7.2 | yes |
| dash | Python 3 library | 1.20.0 | no |
| plotly | Python 3 library | 4.14.3 | no |
The following R libraries are required or recommended. Note the
ewastools library is only required for optional genotype-based shared
identity analyses, which are made as part of the calculated DNAm-based metadata.
| Library | Type | Version | Required? |
|---|---|---|---|
| minfi | R library | 1.32.0 | yes |
| rhdf5 | R library | 2.30.1 | yes |
| DelayedArray | R library | 0.12.3 | yes |
| HDF5Array | R library | 1.14.4 | yes |
| ewastools | R library | 1.7 | no |
Python 2 is only required if you intend to run the MetaSRA-pipeline. For this,
the following libraries are recommended:
| Library | Type | Version | Required? |
|---|---|---|---|
| numpy | Python 2 library | 1.15.4 | no |
| scipy | Python 2 library | 1.1.0 | no |
| scikit-learn | Python 2 library | 0.20.1 | no |
| setuptools | Python 2 library | 0.9.8 | no |
| marisa-trie | Python 2 library | 0.7.5 | no |
| dill | Python 2 library | 0.2.8.2 | no |
| nltk | Python 2 library | 3.4 | no |
First clone the latest version of the recountmethylation_instance repo from GitHub, then
navigate into the directory.
git clone https://github.com/metamaden/recountmethylation_instance
cd recountmethylation_instance
Next, choose a setup option below. Several options have been provided to support various OS environments.
For setup with sh, run the provided script setup_instance.sh:
sh setup_instance.sh
For setup using virtual environments with conda, you may run the script anaconda_setup.sh:
sh anaconda_setup.sh
Alternatively, create the environment from the provided .yml file:
conda env create -f ./inst/yml/environment_rmi_py3.yml
This will set up the main environment, called py3, to run the core data processing rules.
For running MetaSRA-pipeline, you may either set up an additional Python 2 environment or
run the corresponding snakemake rules manually if a Python 2 alias is callable from the
py3 environment.
This tutorial shows how to set up and initiate synchronization of public DNA methylation array data.
Note that most steps involve calling snakemakerules defined in the provided Snakefile script. Logs,
including stdout, for each rule called are stored by default in the "snakemakelogs" subdirectory.
For this example, DNAm array data run using the Illumina Infinium HumanMethylation 450K array platform will be considered. First clone the resource from GitHub, then rename the cloned repo.
git clone https://github.com/metamaden/recountmethylation_instance
mv recountmethylation_instance recountmethylation_instance_hm450k
cd recountmethylation_instance_hm450k
Next, we can perform setup with setup_instance.sh:
sh setup_instance.sh
After successfully completing the above setup steps, the top level of the new instance should look something like this:
Next, we need to configure the instance, including specifying the array platform to target, and specifying sample IDs to exclude.
The HM450K platform is presently targeted by default, but this may change. To explicitly set the platform to target, run the following:
python3 ./recountmethylation_server/src/set_acc.py
This produces the dialogue:
(Y/N) Current target platform accession ID is GPL13534.
Do you want to change the target platform?
Entering Y returns:
(1/2/3) Enter the number of the platform to target:
1. HM450K (GPL13534)
2. EPIC/HM850K (GPL21145)
3. HM27K (GPL8490)
Type 1 to specify the HM450K platform.
Data files are recognized by queries to the GEO DataSets API using Entrez Utilities software. Running a fresh query will identify all valid data files for the targeted platform. To do this, enter:
snakemake --cores 1 new_eqd
The sample/GSM IDs for the most recently available data freezes are included at ./inst/freeze_gsmv/.
Excluding these GSM IDs for this instance will allow us to synchronize the subset of samples available
since the latest data freeze (currently: November 7, 2020).
snakemake --cores 1 exclude_gsm
For this example instance, the rule generated the following output:
Starting with latest detected filter file: recount-methylation-files/equery/gsequery_filt.1618960590
Applying filter...
After filter, retained 72 studies.
Writing new filter file: recount-methylation-files/equery/gsequery_filt.1619031416
Functions downstream will now recognize and use the newer version of the file gsequery_filt.* according
to the newer applied timestamp 1619031416.
Use the following rule to initialize the metadata, including timestamp and version, for this instance:
snakemake --cores 1 new_instance_md
This creates a new subdirectory containing the instance metadata. This is also where the newly generated metadata files will be stored, including metadata mapped from GSM JSON files and DNAm model-based predicted metadata.
This section shows how to download datasets from the GEO servers to an instance. The files of interest
include the SOFT files stored under a unique GSE accession for each study, and a pair of IDAT files
for each unique GSM accession corresponding to a sample. These files are compressed, so you can expect
new downloads to all have a .gz extension.
For example, after successfully downloading a series of study SOFT files, the directory at
./recount-methylation-files/gse_soft/ might look like this:
And the directory at ./recount-methylation-files/idats/ might look something like this:
recountmethylation_instance uses the Entrez Direct queries in the previous section to make URLs
to download study and sample datasets from the GEO servers. Running the server is a safe way to
ensure the correct files are downloaded and that downloaded files are tracked. If the server will
not run continuously, or repeated attempts to complete server runs fail, you can still download the
files using the provided .R scripts.
Before we can start downloading public data, we need to run the MongoDB service with sudo access. This can be done with either:
service mongod start
or
sudo service mongod start
Once MongoDB is running, we can initialize the server with:
snakemake --cores 1 run_server
If this rule hangs, you may alternatively call the script manually:
python3 ./recountmethylation_server/src/server.py
The server.py script process will systematically target and download study SOFT files and sample IDAT files,
according to the contents of the filtered EDirect query files.
Note, you may need to restart the server process periodically if your connection is interrupted, the MongoDB service
stops, etc. To avoid repeated hanging on corrupt or malformed files, target study/GSE ids are shuffled for
each server.py run.
As the server process runs, you may monitor its progress. Check the total study SOFT and sample IDAT files downloaded with:
ls -l recount-methylation-files/gse_soft/ | wc -l
ls -l recount-methylation-files/idats/ | wc -l
For added convenience, a server dashboard utility has been provided. This displays the instance files over time, and allows you to track the addition of new files over time. Run the dashboard with:
snakemake --cores 1 server_dash
Entering the displayed IP address into a browser tab will display the server dashboard.
An alternative method of obtaining study and sample data from the GEO servers is to use the provided
.R scripts. These scripts compare downloaded files to the full set of study/GSE and sample/GSM IDs,
and they will try downloading data for remaining IDs. To download remaining IDATs, use:
Rscript ./inst/scripts/dl_idats.R
To download SOFT files, run:
Rscript ./inst/scripts/dl_soft.R
Once data files have been downloaded, they can be prepared for compilation.
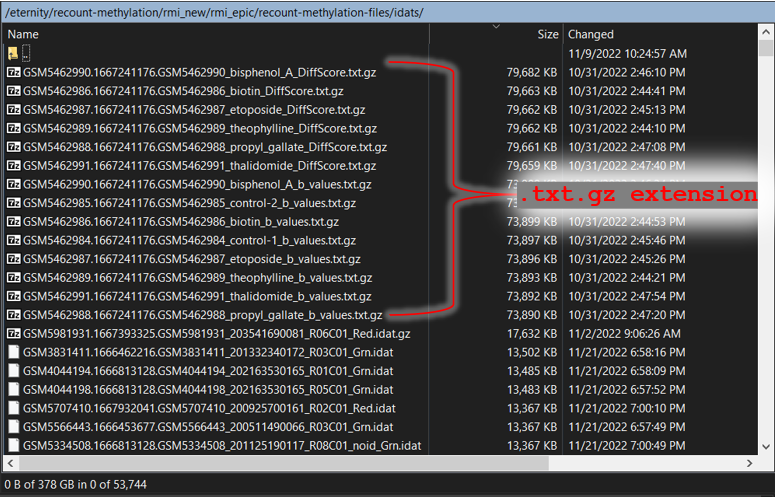
Over the course of the following steps, you may find unexpected file types have been downloaded, such as files with
.txt.gz extension that were downloaded to the ./recount-methylation-files/idats folder (see below). These types of
anomalies are to be expected when performing a comprehensive acquisition and compilation of public data.
The instance will be able to handle many of these types of anomalies, however it is still possible new anomalous filenames and
extensions can be encountered whenever new public data is compiled. It is entirely up to you, the user, to decide how to handle
files in these situations. For instance, you may choose to manually delete these files, or alternatively you could move them to
a new location (e.g. move .txt files to a new directory at ./recount-methylation-files/txt/) and then target them in a future
compilation.
The sample IDATs are paired files containing red and green signals from the array runs. Thus, two
valid IDATs are expected per sample/GSM id, where their filenames end as .*_Red.idat.* and .*_Grn.idat.*.
Since we download the IDATs as compressed .gz files, we need to expand them.
snakemake --cores 1 unzip_idats
Because paired IDATs may have distinct filename timestamps, it is necessary to create hardlinks to ensure timestamps match for sample IDAT pairs.
snakemake --cores 1 make_idat_hlinks
Once the IDATs have been prepared, they can be compiled into various database file types. To begin,
read the raw/unnormalized red and green signals are into flat compilation tables with get_rg_compilations.
snakemake --cores 1 get_rg_compilations
Since downloaded IDATs may occasionally be malformed, mislabeled, or otherwise unreadable, a series of checks are performed automatically, including an evaluation of file sizes and similar sizes for paired red and green signal files. In practice, this means an initial attempt is made to read in samples as batches, or chunks. If the attempt to read a given chunk fails, individual sample IDATs are read in successively and combined, and invalid files are excluded.
Next, the flat red and green signal compilation tables are used to make HDF5 h5 and HDF5-SummarizedExperiment
h5se objects.
snakemake --cores 1 get_h5db_rg
snakemake --cores 1 get_h5se_rg
Once the red and green RGChannelSet tables and database files are generated, you may proceed to generate
additional compilation files. The full list of compilation rules, in their recommended order, is as follows:
get_rg_compilations: Make flat tables containing the Red and Green signal intensities.get_h5db_rg: Make an HDF5 database.h5file containing the Red and Green signal intensities.get_h5se_rg: Make an HDF5-SummarizedExperimenth5sefile containing the Red and Green signal intensitiesget_h5db_gm: Make an HDF5 database.h5file containing the Methylated and Unmethylated signals.get_h5se_gm: Make an HDF5-SummarizedExperimenth5sefile containing the Methylated and Unmethylated signals.get_h5db_gr: Make an HDF5 database.h5file containing the Beta-values (DNAm fractions).get_h5se_gr: Make an HDF5-SummarizedExperimenth5sefile containing the Beta-values (DNAm fractions).
You could run the above from an active R session by running R and then entering the following:
library(recountmethylation.pipeline)
get_rg_dtables()
get_h5db_rg()
get_h5se_rg()
get_h5db_gm()
get_h5se_gm()
get_h5db_gr()
get_h5se_gr()
You could also run the following one-liner from your Terminal to call the steps in their recommended order:
snakemake --cores 1 run_dnam_pipeline
Sample metadata is contained in the SOFT files. After expanding the .gz compressed SOFT files, we need to
extract the sample-specific metadata into .json files before mapping with either MetaSRA-pipeline or the
included mapping scripts. After extraction, the .json files are further filtered to remove study-specific
metadata.
snakemake --cores 1 process_soft
snakemake --cores 1 apply_jsonfilt
As an example, SOFT-extracted metadata for GSM4671807
would be stored at ./gsm_soft/ and look something like:
!Sample_title = K562 - control 1
!Sample_geo_accession = GSM4671807
!Sample_status = Public on Nov 23 2022
...
...
!Sample_characteristics_ch1 = cell line: K562
!Sample_characteristics_ch1 = disease: chronic myelogenous leukemia
!Sample_characteristics_ch1 = treatment: control
By contrast, the JSON-formatted version of GSM4671807's metadata would be stored at ./gsm_json/ and appear as:
[
{
"!Sample_title": "K562 - control 1",
"!Sample_geo_accession": "GSM4671807",
"!Sample_status": "Public on Nov 23 2022",
"!Sample_submission_date": "Jul 15 2020",
"!Sample_last_update_date": "Nov 23 2022",
"!Sample_type": "genomic",
"!Sample_channel_count": "1",
"!Sample_source_name_ch1": "control replicate 1",
"!Sample_organism_ch1": "Homo sapiens",
"!Sample_taxid_ch1": "9606",
"!Sample_characteristics_ch1": "cell line: K562",
"!Sample_characteristics_ch1.1": "disease: chronic myelogenous leukemia",
"!Sample_characteristics_ch1.2": "treatment: control",
"!Sample_treatment_protocol_ch1": "GSE154471\n",
"cell_line": "K562",
"treatment": "control"
}
]
And finally the filtered JSON file for GSM4671807, stored at ./gsm_jsonfilt/, looks like:
[
{
"!Sample_characteristics_ch1": "cell line: K562",
"!Sample_characteristics_ch1.1": "disease: chronic myelogenous leukemia",
"!Sample_characteristics_ch1.2": "treatment: control",
"!Sample_source_name_ch1": "control replicate 1",
"!Sample_title": "K562 - control 1"
}
]
This JSON formatting is important because it allows the file to be read by the MetaSRA-pipeline. We also
apply a filter on metadata fields such as !Sample_treatment_protocol_ch1 which are likely to be repeated
across samples. This can help ensure the pipeline maps characteristics which differentiate samples within
the same experiment, rather than mapping the same terms to every sample in a given experiment.
Once the .json files with sample-specific metadata have been prepared, you have the option of running
any of the following available metadata processing rules:
do_mdmap: Map and harmonize metadata using the provided scripts. These scripts use regular expressions to automatically detect and categorize tags in.jsonfiles, and then to uniformly format and annotate metadata terms under several columns, including "disease" (e.g. disease condition or experiment group), "tissue" (e.g. tissue of origin), "age" (chronological age), and "sex" (provided sex information).run_msrap: Run the MetaSRA-pipeline. This produces sample type predictions, as well as ENCODE ontology terms from several major ontology dictionaries.do_dnam_md: Get DNAm-derived metadata (model-based predictions for age, sex, and blood cell types) and quality metrics (BeadArray controls, methylated signal, unmethylated signal, and predictated replicates). This should be run after all 3 types of h5se compilations are complete.
Once one or all of these rules have been successfully run, compile and append the harmonized metadata to the available DNAm data compilations:
snakemake --cores 1 make_md_final
snakemake --cores 1 append_md
You could also accomplish the above by running R and then entering the following:
library(recountmethylation.pipeline)
get_mdmap()
get_mddnam()
md_agg()
append_md_snakemake()
If you find recountmethylation_instance useful, the following resources may also be helpful.
-
recountmethylation. R/Bioconductor package providing utilities to access and analyze public DNAm array data from GEO. 10.18129/B9.bioc.recountmethylation -
minfi. R/Bioconductor package for analysis of DNA methylation arrays. 10.18129/B9.bioc.minfi -
HDF5Array. R/Bioconductor package for handling HDF5 and HDF5SummarizedExperiment objects with DelayedArray-powered backends. 10.18129/B9.bioc.HDF5Array
-
Sean K Maden, Reid F Thompson, Kasper D Hansen, Abhinav Nellore, Human methylome variation across Infinium 450K data on the Gene Expression Omnibus, NAR Genomics and Bioinformatics, Volume 3, Issue 2, June 2021, https://doi.org/10.1093/nargab/lqab025
-
Timothy J Triche Jr, Daniel J Weisenberger, David Van Den Berg, Peter W Laird, Kimberly D Siegmund, Low-level processing of Illumina Infinium DNA Methylation BeadArrays, Nucleic Acids Research, Volume 41, Issue 7, April 2013, https://doi.org/10.1093/nar/gkt090