Authors:
- Emily Alsentzer (Equal contribution)
- Michelle M. Li (Equal contribution)
- Shilpa N. Kobren
- Ayush Noori
- Undiagnosed Diseases Network
- Isaac S. Kohane
- Marinka Zitnik
Additional resources:
- Paper
- Project Website
- HuggingFace Space illustrating SHEPHERD's use for causal gene nomination, patients-like-me identification and disease characterization
There are over 7,000 unique rare diseases, some of which affect 3,500 or fewer patients in the US. Due to clinicians' limited experience with such diseases and the considerable heterogeneity of their clinical presentations, many patients with rare genetic diseases remain undiagnosed. While artificial intelligence has demonstrated success in assisting diagnosis, its success is usually contingent on the availability of large annotated datasets. Here, we present SHEPHERD, a deep learning approach for multi-faceted rare disease diagnosis. To overcome the limitations of supervised learning, SHEPHERD performs label-efficient training by (1) training exclusively on simulated rare disease patients without the use of any real labeled data and (2) incorporating external knowledge of known phenotype, gene and disease associations via knowledge-guided deep learning.
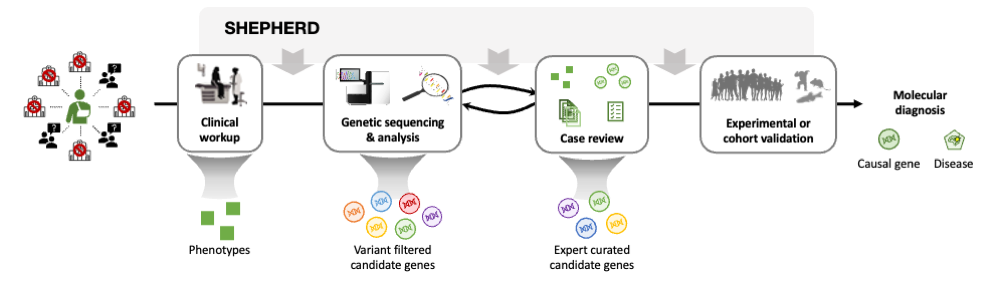
After years of failed diagnostic attempts, once a patient is accepted to the UDN, they receive a thorough clinical workup and genetic sequencing, and their case is analyzed in an iterative process to identify the candidate genes likely to explain the patient's symptoms. SHEPHERD can be utilized throughout the pipeline to accelerate the diagnosis process: after the clinical workup to find similar patients, after the sequencing analysis to identify strong candidate genes, and after case review to further prioritize candidate genes, characterize the patient's disease, and/or validate candidate genes by finding phenotype and genotype-matched patients.
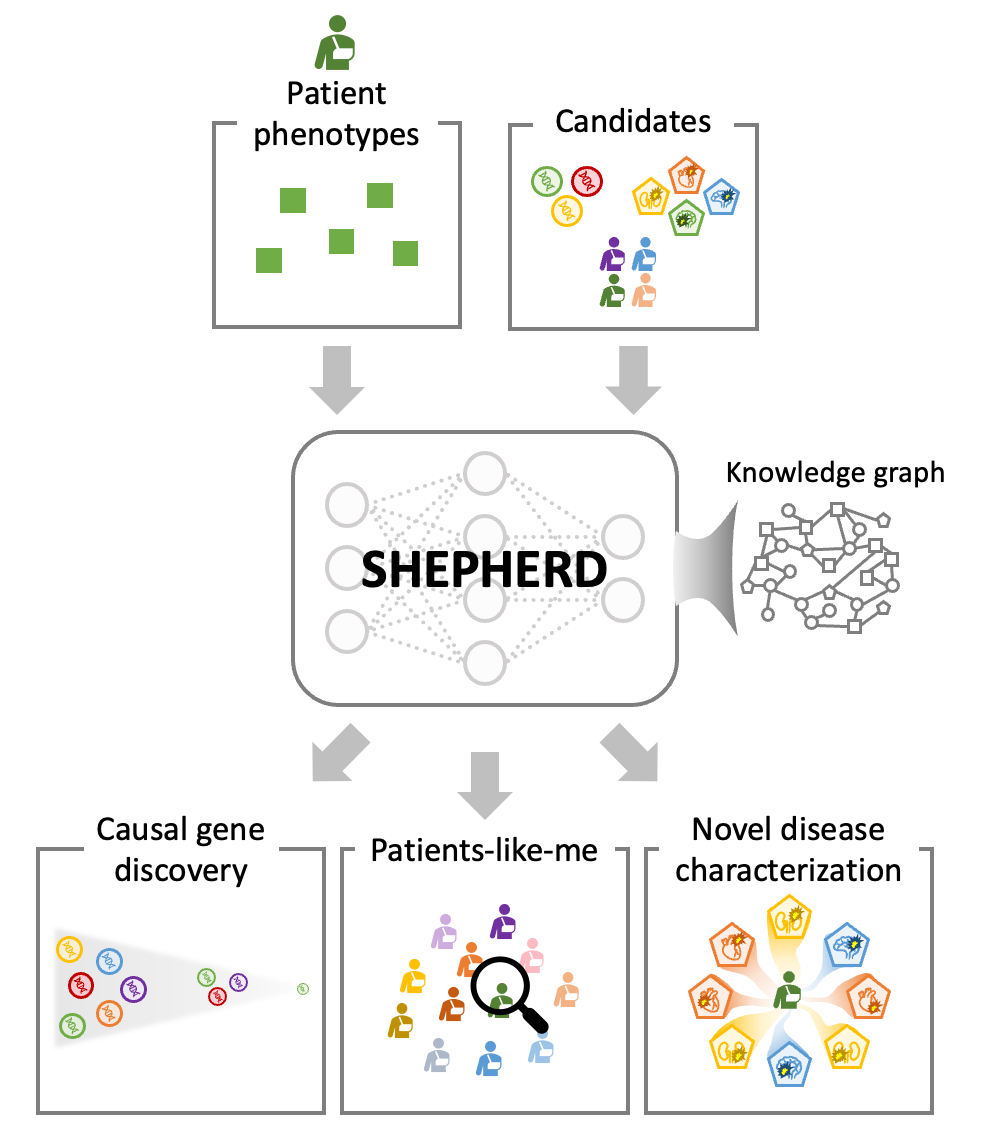
SHEPHERD is guided by existing knowledge of diseases, phenotypes, and genes to learn novel connections between a patient's clinico-genomic information and phenotype and gene relationships. SHEPHERD takes in as input the patient’s set of phenotypes as well a list of either candidates genes, patients, or diseases and leverages an external rare disease knowledge graph to perform multi-faceted rare disease diagnosis: causal gene discovery, patients-like-me identification, and novel disease characterization.
First, clone the GitHub repository:
git clone https://github.com/mims-harvard/SHEPHERD
cd SHEPHERD
This codebase leverages Python, Pytorch, Pytorch Geometric, etc. To create an environment with all of the required packages, please ensure that conda is installed and then execute the commands:
conda env create -f environment.yml
conda activate shepherd
bash install_pyg.sh
The data is hosted on Harvard Dataverse. To maintain the directory structure while downloading the files, make sure to select all files and download in the original format. Make sure to also unzip all files in the download (e.g. this file)
We provide the following datasets for training SHEPHERD:
- Rare disease knowledge graph
- Disease-split train and validation sets for simulated patients
- MyGene2 patients
More details about the simulated rare disease patients can be found here. We are unfortunately unable to provide the UDN patients due to patient privacy concerns.
The rare disease knowledge graph and patient datasets are provided in the appropriate format for SHEPHERD. If you would like to add your own set of patients, please adhere to the format used in the MyGene2 and simulated rare disease patients' files (see README in data_prep folder for more details). The file should be structured as a jsonlines file, where each json (i.e., line in the file) contains information for a single patient. Each json must contain at least the following elements: patient ID ("id"), a list of phenotypes present in the patient as HPO terms ("positive_phenotypes"), and a list of causal genes as Ensembl IDs ("true_genes"). To run causal gene discovery, the json must also include a list of all candidate genes as Ensembl IDs ("all_candidate_genes"). To run novel disease characterization, the json must also include a list of true disease names as MONDO IDs ("true_diseases").
Go to project_config.py and set the project directory (PROJECT_DIR) to be the path to the data folder downloaded in the previous step.
If you would like to use your own data, be sure to
- Modify the data variables in
project_config.pyin lines 10-16. - Generate the required shortest path length data files for your patients using the code and instructions in
data_prep/shortest_paths
We also provide checkpoints for SHEPHERD after pretraining and after training on the rare disease diagnosis tasks. The checkpoints for SHEPHERD can be found here. You'll need to move them to the directory specified by project_config.PROJECT_DIR / 'checkpoints' (see above step). Make sure all downloaded files are unzipped. You can use these checkpoints directly with the predict.py scripts below instead of training the models yourself.
You can run SHEPHERD on your own patient cohort by using our provided model checkpoints (i.e., no re-training needed). Please review this README to learn how to preprocess and run SHEPHERD on your own patient dataset.
You can reproduce our pretraining results or pretrain SHEPHERD on your own knowledge graph:
cd shepherd
python pretrain.py \
--edgelist KG_edgelist_mask.txt \
--node_map KG_node_map.txt \
--save_dir checkpoints/
To see and/or modify the default hyperparameters, please see the get_pretrain_hparams() function in shepherd/hparams.py.
An example bash script is provided in shepherd/run_pretrain.sh.
✨ To train SHEPHERD for causal gene discovery:
cd shepherd
python train.py \
--edgelist KG_edgelist_mask.txt \
--node_map KG_node_map.txt \
--patient_data disease_simulated \
--run_type causal_gene_discovery \
--saved_node_embeddings_path checkpoints/<BEST_PRETRAIN_CHECKPOINT>.ckpt
An example bash script is provided in shepherd/run_causal_gene_discovery.sh.
✨ To train SHEPHERD for patients-like-me identification:
cd shepherd
python train.py \
--edgelist KG_edgelist_mask.txt \
--node_map KG_node_map.txt \
--patient_data disease_simulated \
--run_type patients_like_me \
--saved_node_embeddings_path checkpoints/<BEST_PRETRAIN_CHECKPOINT>.ckpt
An example bash script is provided in shepherd/run_patients_like_me.sh.
✨ To train SHEPHERD for novel disease characterization:
cd shepherd
python train.py \
--edgelist KG_edgelist_mask.txt \
--node_map KG_node_map.txt \
--patient_data disease_simulated \
--run_type disease_characterization \
--saved_node_embeddings_path checkpoints/<BEST_PRETRAIN_CHECKPOINT>.ckpt
An example bash script is provided in shepherd/run_disease_characterization.sh.
To see and/or modify the default hyperparameters, please see the get_train_hparams() function in shepherd/hparams.py.
After training SHEPHERD, you can calculate SHEPHERD's performance on a test patient dataset. Simply run the same command used to train the model with the additional flags: --do_inference and --best_ckpt <PATH/TO/BEST_MODEL_CHECKPOINT.ckpt>.
After training SHEPHERD (you may also simply use our already-trained models), you can generate predictions for patients (without performance metrics). An example bash script can be found here.
The results of the predict.py script are found in
project_config.PROJECT_RESULTS/<TASK>/<RUN_NAME>/<DATASET_NAME>
where
<TASK>iscausal_gene_discovery,patients_like_me, ordisease_characterization<RUN_NAME>is the name of the run created during training<DATASET_NAME>is the name of your patient cohort
✨ To run causal gene discovery:
cd shepherd
python predict.py \
--run_type causal_gene_discovery \
--patient_data <TEST_DATA> \
--edgelist KG_edgelist_mask.txt \
--node_map KG_node_map.txt \
--saved_node_embeddings_path checkpoints/<BEST_PRETRAIN_CHECKPOINT>.ckpt \
--best_ckpt PATH/TO/BEST_MODEL_CHECKPOINT.ckpt
To generate predictions on your own dataset, please use --patient_data my_data. To generate predictions on simulated test patients, please use --patient_data test_predict. If using the provided checkpoint models, checkpoints/<BEST_PRETRAIN_CHECKPOINT>.ckpt should be checkpoints/pretrain.ckpt and PATH/TO/BEST_MODEL_CHECKPOINT.ckpt should be checkpoints/causal_gene_discovery.ckpt.
✨ To run patients-like-me identification:
cd shepherd
python predict.py \
--run_type patients_like_me \
--patient_data <TEST_DATA> \
--edgelist KG_edgelist_mask.txt \
--node_map KG_node_map.txt \
--saved_node_embeddings_path checkpoints/<BEST_PRETRAIN_CHECKPOINT>.ckpt \
--best_ckpt PATH/TO/BEST_MODEL_CHECKPOINT.ckpt
To generate predictions on your own dataset, please use --patient_data my_data. To generate predictions on simulated test patients, please use --patient_data test_predict. If using the provided checkpoint models, checkpoints/<BEST_PRETRAIN_CHECKPOINT>.ckpt should be checkpoints/pretrain.ckpt and PATH/TO/BEST_MODEL_CHECKPOINT.ckpt should be checkpoints/patients_like_me.ckpt.
✨ To run novel disease characterization:
cd shepherd
python predict.py \
--run_type disease_characterization \
--patient_data <TEST_DATA> \
--edgelist KG_edgelist_mask.txt \
--node_map KG_node_map.txt \
--saved_node_embeddings_path checkpoints/<BEST_PRETRAIN_CHECKPOINT>.ckpt \
--best_ckpt PATH/TO/BEST_MODEL_CHECKPOINT.ckpt
To generate predictions on your own dataset, please use --patient_data my_data. To generate predictions on simulated test patients, please use --patient_data test_predict. If using the provided checkpoint models, checkpoints/<BEST_PRETRAIN_CHECKPOINT>.ckpt should be checkpoints/pretrain.ckpt and PATH/TO/BEST_MODEL_CHECKPOINT.ckpt should be checkpoints/disease_characterization.ckpt.
To see and/or modify the default hyperparameters, please see the get_predict_hparams() function in shepherd/hparams.py.
@article{shepherd,
title={Few shot learning for phenotype-driven diagnosis of patients with rare genetic diseases},
author={Alsentzer, Emily and Li, Michelle M. and Kobren, Shilpa and Noori, Ayush and Undiagnosed Diseases Network and Kohane, Isaac S. and Zitnik, Marinka},
journal={medRxiv},
year={2024}
}
Please leave a Github issue or contact Emily Alsentzer at ealsentzer@bwh.harvard.edu and Michelle Li at michelleli@g.harvard.edu.