Single-cell / single nuclei ATAC-seq pipeline
scATAC is made of following steps:
- decomplex scATAC-seq data by scATAC_debarcode [OPTIONAL];
- map using bowtie2 followed by filtering reads with MAPQ < 30; (currently using bowtie2)
- correct barcode error caused by sequencing error by allowing certain number of mismatches [2];
- split reads to individual cells based on the barcode combination;
- remove PCR duplication for each cell; (currently using
samtools rmdup) - merge reads from different cells;
- generate barcode frequency table;
- filter cells with reads counts less than given number [500];
- summerize and generate a log file;
!!! For the old version of the shell scripts, check the legend README here. Below is the use of this pipeline wrappered using bigdatascript (bds) language.
Installation
For tscc user
No installation is necessary. The dependent softwares and libs are installed in a conda enrionment that can directly be loaded.
Add the following into your ~/.bashrc. Then . ~/.bashrc and test by using scATAC.bds -help,
export PATH=/usr/lib/jvm/java-1.8.0-openjdk-1.8.0.31-1.b13.el6_6.x86_64/bin:$PATH
export PATH="$PATH:/projects/ps-epigen/software/miniconda3/bin/"
export _JAVA_OPTIONS="-Xms256M -Xmx728M -XX:ParallelGCThreads=1"
export PATH="$PATH:/projects/ps-epigen/software/.bds/"
export PATH="$PATH:/projects/ps-epigen/software/scATAC/bin"
export PICARDROOT="/projects/ps-epigen/software/miniconda3/envs/bds_atac/share/picard-1.126-4/"For other user
- Install conda: check https://github.com/kundajelab/atac_dnase_pipelines#conda
- install bds: check https://github.com/kundajelab/atac_dnase_pipelines#bigdatascript
- Run
bash ./install_dependencies.shto generatebds_scATACenvironment to encapsulate dependent softwares.
Usage
$ conda activate bds_scATAC # activate conda env
$ scATAC.bds -help
Picked up _JAVA_OPTIONS: -Xms256M -Xmx728M -XX:ParallelGCThreads=1
== scATAC pipeline settings
-barcode_dir <string> : folder that contains r7_ATAC, i7_ATAC, i5_ATAC and r5_ATAC barcode.
-bowtie2_idx <string> : Bowtie2 indexed reference genome.
-mark_duplicate <string> : path to picard MarkDuplicates.jar [MarkDuplicates.jar].
-max_barcode_mismatch <int> : max barcode mismatch allowed for barcode error correction [2].
-min_read <int> : cells with reads less than 'min_read' will be filtered [500].
-prefix <string> : prefix of output files.
-r1 <string> : fastq.gz file that contains forward reads (only .gz allowed).
-r2 <string> : fastq.gz file that contains reverse reads (only .gz allowed).
-threads <int> : Set threads for Bowtie2 mapping, sorting and duplication removal [1].For tscc user
Check the pbs files in the \examples folder.
Input
Currently it run start from the fastq files after decomplex and demultiplex.
Output
- scATAC generates two files '.log' and '.bam'. '.bam' is the final file that contains all usable reads and '.log' includes data metrics.
- Also, pipeline will generate a report html named like
scATAC.bds.20171028_022747_846.report.*. It contains all the information about your run.
Features
- Timeline for each run. An example timeline in your report:
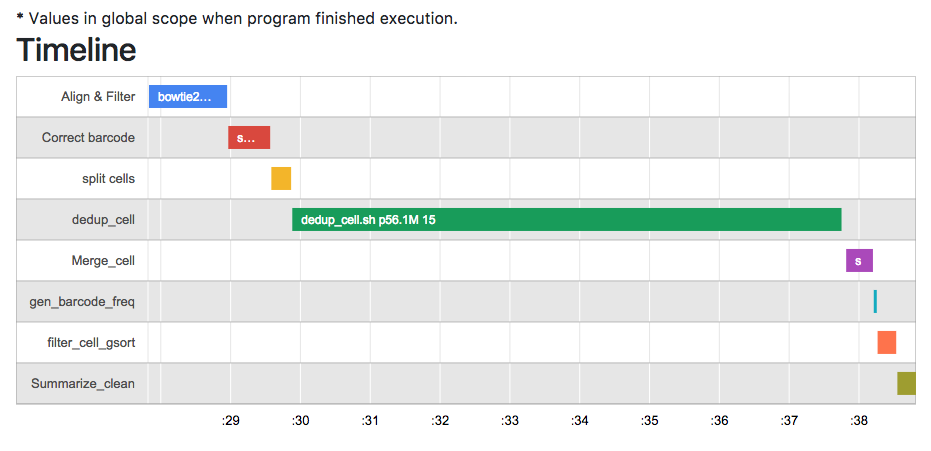
- Detailed reports on each steps, including cmd, input, output and stderr and etc.
- Resume from last failed point just rerun the script.
- Through
conda, it is easy to be installed in any machine.
Licence
MIT