Badread is a long-read simulator tool that makes – you guessed it – bad reads! It can imitate many kinds of problems one might encounter in real long-read sets: chimeras, low-quality regions, systematic basecalling errors and more.
Badread does not try to be best at imitating real reads (though it's not too bad, see this comparison between Badread and other long-read simulators). Rather, it was intended to give users control over the quality of its simulated reads. I made Badread for the purpose of testing tools which take long reads as input. With it, one can increase the rate of different types of read problems, to see what effect it has.
Badread is published in the Journal of Open Source Software. If you use it in your research, please cite this manuscript:
Wick RR. Badread: simulation of error-prone long reads. Journal of Open Source Software. 2019;4(36):1316. doi:10.21105/joss.01316.
Badread runs on MacOS and Linux. It may not work natively on Windows (I haven't tried) but can be run using the Windows subsystem for Linux. It requires Python 3.6 or later.
To install Badread you'll need pip and Git. It also uses a few Python packages (Edlib, NumPy, SciPy and Matplotlib) but these should be taken care of by the installation process.
You can install Badread using pip, either from a local copy:
git clone https://github.com/rrwick/Badread.git
pip3 install ./Badread
badread --helpOr directly from GitHub:
pip3 install git+https://github.com/rrwick/Badread.git
badread --helpIf these installation commands aren't working for you (e.g. an error message like Command 'pip3' not found or command 'gcc' failed with exit status 1) then check out the installation issues page on the wiki.
Badread can also be run directly from its repository by using the badread-runner.py script, no installation required:
git clone https://github.com/rrwick/Badread.git
Badread/badread-runner.py -hIf you run Badread this way, it's up to you to make sure that all necessary Python packages are installed.
If you need a reference genome to try out Badread, you can download this file which is an assembly of the Klebsiella pneumoniae SGH10 genome – a nasty hypervirulent strain (read more about it here).
Badread's default settings correspond to Oxford Nanopore reads of mediocre quality:
badread simulate --reference ref.fasta --quantity 50x \
| gzip > reads.fastq.gz
Alternatively, you can use Badread's built-in models to imitate PacBio reads. This command also adjusts the identity and length distributions to be a bit more PacBio-like:
badread simulate --reference ref.fasta --quantity 50x \
--error_model pacbio --qscore_model pacbio --identity 85,95,3 --length 7500,7500 \
| gzip > reads.fastq.gz
Very bad reads:
badread simulate --reference ref.fasta --quantity 50x --glitches 1000,100,100 \
--junk_reads 5 --random_reads 5 --chimeras 10 --identity 75,90,8 \
| gzip > reads.fastq.gz
Very nice reads:
badread simulate --reference ref.fasta --quantity 50x --error_model random \
--qscore_model ideal --glitches 0,0,0 --junk_reads 0 --random_reads 0 \
--chimeras 0 --identity 95,100,4 --start_adapter_seq "" --end_adapter_seq "" \
| gzip > reads.fastq.gz
Badread simulates reads by mimicking the process of real sequencing: breaking the DNA into fragments, adding adapters and then reading the fragments into nucleotide sequences.
Here is an overview of how Badread makes each of its reads:
-
Use the fragment length distribution to choose a length for the read.
-
Choose a type of fragment:
- Most will be fragments of sequence from the reference FASTA. These are equally likely to come from either strand, and can loop around circular references. If there are multiple reference sequences with different depths, then the likelihood of the fragment coming from each sequence is proportional to that sequence's depth.
- Depending on the settings, some fragments may also be junk or random sequence.
-
Add adapter sequences to the start and end of the fragment, based on the adapter settings.
-
As determined by the chimera rate, there is a chance that Badread will make another fragment and concatenate it onto the current fragment (possibly with adapter sequences in between, possibly not).
-
Add glitches to the fragment, based on the glitch settings.
-
Choose a percent identity for the read using the read identity distribution.
-
'Sequence' the fragment by adding errors until it has the target percent identity.
- Errors are chosen using the error model and are added at random positions in the read.
- This step performs periodic alignments between the original fragment and the error-added sequence, so Badread can track the read's actual identity. This allow it to be precise (if Badread is aiming for a 91.5% identity read, it will be very close to 91.5% identity) but slow. If you find that Badread is too slow, check out the wiki page on running it in parallel.
-
Generate quality scores for each base using the qscore model.
-
Output the read and quality in FASTQ format.
usage: badread simulate --reference REFERENCE --quantity QUANTITY [--length LENGTH]
[--identity IDENTITY] [--error_model ERROR_MODEL]
[--qscore_model QSCORE_MODEL] [--seed SEED] [--start_adapter START_ADAPTER]
[--end_adapter END_ADAPTER] [--start_adapter_seq START_ADAPTER_SEQ]
[--end_adapter_seq END_ADAPTER_SEQ] [--junk_reads JUNK_READS]
[--random_reads RANDOM_READS] [--chimeras CHIMERAS] [--glitches GLITCHES]
[--small_plasmid_bias] [-h] [--version]
Generate fake long reads
Required arguments:
--reference REFERENCE Reference FASTA file (can be gzipped)
--quantity QUANTITY Either an absolute value (e.g. 250M) or a relative depth (e.g. 25x)
Simulation parameters:
Length and identity and error distributions
--length LENGTH Fragment length distribution (mean and stdev, default: 15000,13000)
--identity IDENTITY Sequencing identity distribution (mean, max and stdev, default:
85,95,5)
--error_model ERROR_MODEL Can be "nanopore", "pacbio", "random" or a model filename (default:
nanopore)
--qscore_model QSCORE_MODEL Can be "nanopore", "pacbio", "random", "ideal" or a model filename
(default: nanopore)
--seed SEED Random number generator seed for deterministic output (default:
different output each time)
Adapters:
Controls adapter sequences on the start and end of reads
--start_adapter START_ADAPTER Adapter parameters for read starts (rate and amount, default: 90,60)
--end_adapter END_ADAPTER Adapter parameters for read ends (rate and amount, default: 50,20)
--start_adapter_seq START_ADAPTER_SEQ
Adapter sequence for read starts (default:
AATGTACTTCGTTCAGTTACGTATTGCT)
--end_adapter_seq END_ADAPTER_SEQ
Adapter sequence for read ends (default: GCAATACGTAACTGAACGAAGT)
Problems:
Ways reads can go wrong
--junk_reads JUNK_READS This percentage of reads will be low-complexity junk (default: 1)
--random_reads RANDOM_READS This percentage of reads will be random sequence (default: 1)
--chimeras CHIMERAS Percentage at which separate fragments join together (default: 1)
--glitches GLITCHES Read glitch parameters (rate, size and skip, default: 10000,25,25)
--small_plasmid_bias If set, then small circular plasmids are lost when the fragment
length is too high (default: small plasmids are included regardless
of fragment length)
Other:
-h, --help Show this help message and exit
--version Show program's version number and exit
The reference genome must be given as a FASTA file (either gzipped or not) using the --reference argument.
Each sequence's depth can be specified in the FASTA header, e.g. using depth=1.1 or depth=15. Badread will use this to determine the relative abundance of each sequence. This can be useful for both bacterial genomes (where plasmids may be higher depth than the chromosome) and eukaryote genomes (where chloroplast/mitochondrial genomes may be higher depth than the rest of the genome).
Circular sequences are indicated by including circular=true in the FASTA header. This allows reads to loop past the end and back to the start of the sequence.
For a couple of examples, check out the reference FASTA page on the wiki.
Badread generates fragment lengths from a gamma distribution. While a gamma distribution is usually parameterised with shape and scale (k and θ) or shape and rate (α and β), I don't find these particularly intuitive. So Badread instead defines the fragment lengths using mean and standard deviation.
There are two ways to think about fragment lengths: the distribution of the fragment lengths and the distribution of bases in the fragments. The latter distribution is higher because larger fragments contribute more bases. The read N50 is the median of the base (red) distribution – half the bases will be in reads shorter than this and half in longer reads.
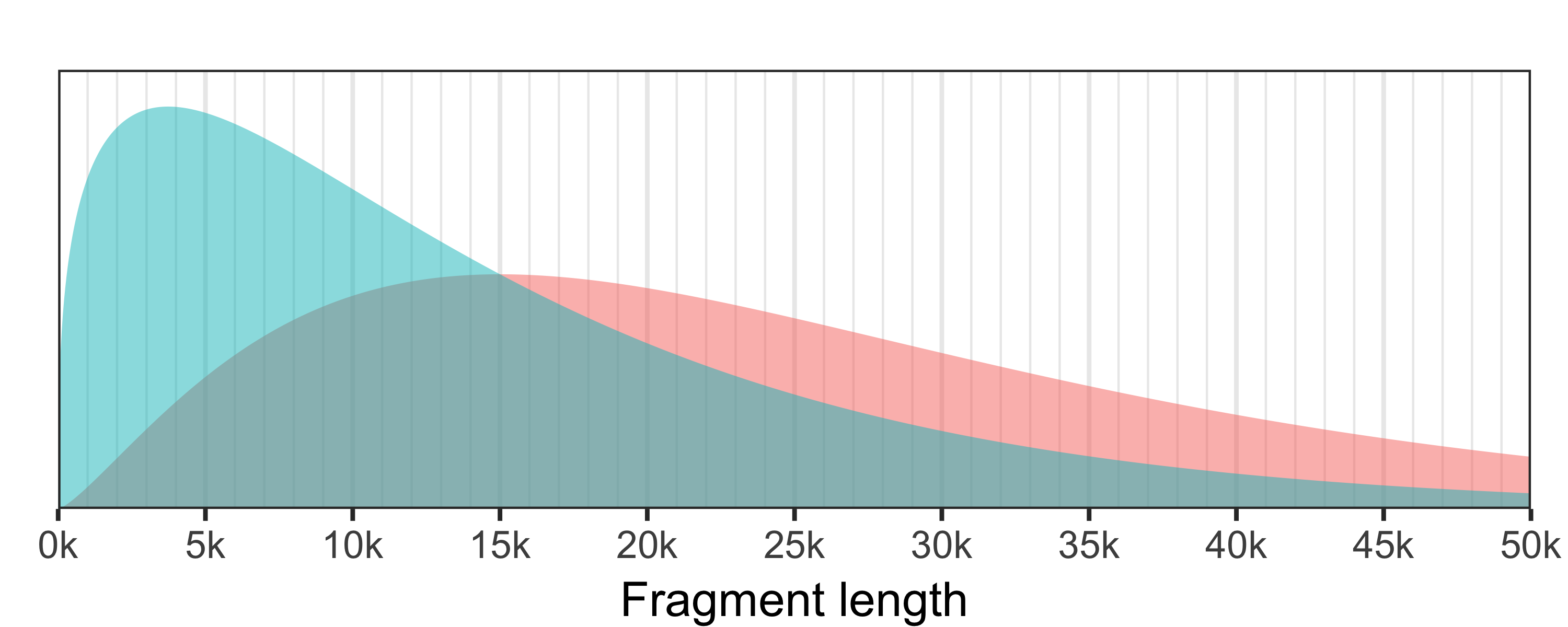 Badread's default is
Badread's default is --length 15000,13000 (mean=15000, stdev=13000) which corresponds to a decent Nanopore run (N50=22.6 kbp). The fragment length distribution is in blue, while the base distribution is in red.To see the equations and interactively explore how different parameters affect the distributions, check out this Desmos plot. |
Note that these parameters control the length of the fragments, not the final reads. These differ because: adapters are added to fragments, glitches can lengthen/shorten fragments, adding read errors can change the length (especially if the error model is biased towards insertions or deletions) and chimeras are made by concatenating multiple fragments together.
Badread generates read identities from a beta distribution. Like with fragment lengths, Badread defines the distribution with mean and standard deviation instead of using the more formal (and less intuitive) shape parameters (α and β). In addition, Badread scales the distribution down using a maximum value, for a total of three parameters: mean,max,stdev.
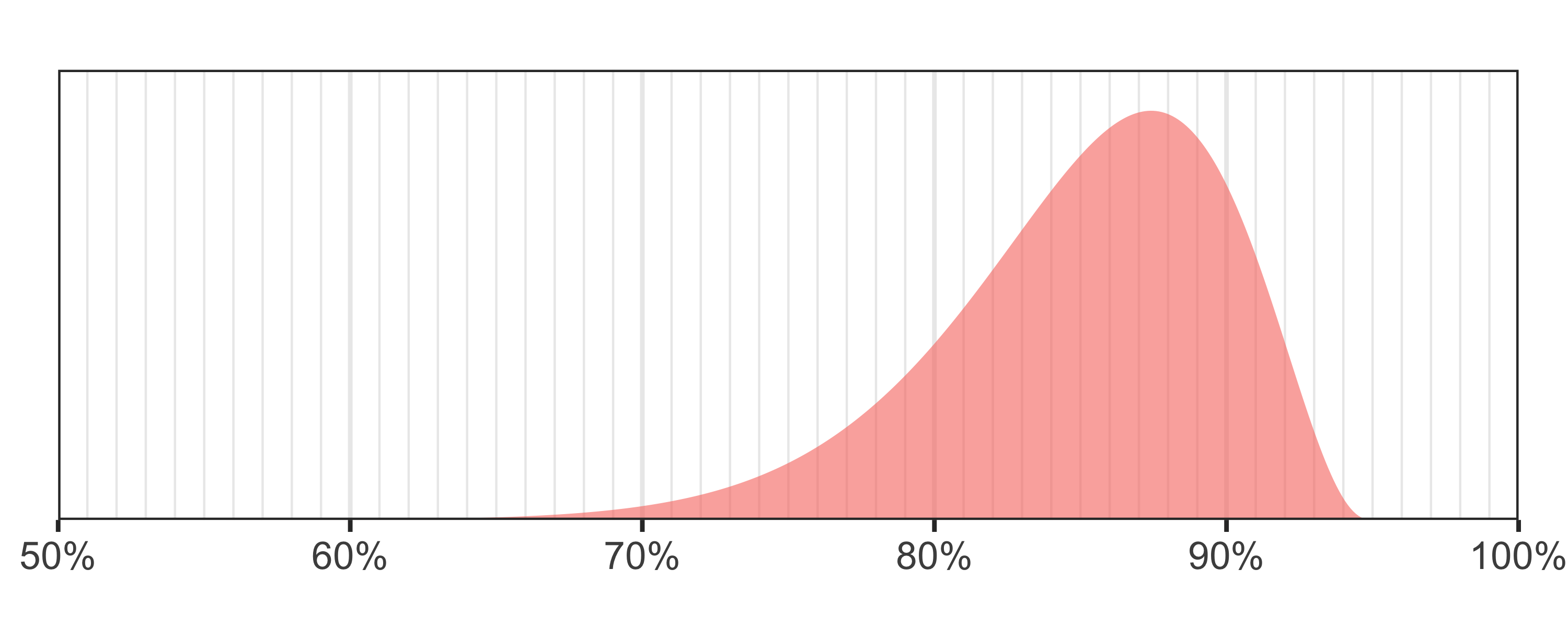 Badread's default is
Badread's default is --identity 85,95,5 which correspond to an okay (but not great) Nanopore sequencing run.To see the equations and interactively explore how different parameters affect the distribution, check out this Desmos plot. |
For detail on how Badread defines identity, check out this page on the wiki.
The possible values for the --error_model argument are:
nanopore: a model trained on real Nanopore reads (the default)pacbio: a model trained on real PacBio readsrandom: a random error model with 1/3 chance each of insertion, deletion and substitution- a file path for a trained model
For more information on how error models work, see this page on the wiki. For instructions on building your own error model, see this page.
The possible values for the --qscore_model argument are:
nanopore: a model trained on real Nanopore reads (the default)pacbio: a model trained on real PacBio readsrandom: a model where qscores are meaningless and give no indication of read/base qualityideal: a model where scores are unrealistically informative about read/base quality- a file path for a trained model
For more information on how qscore models work, see this page on the wiki. For instructions on building your own qscore model, see this page.
Adapter sequences are controlled with the --start_adapter_seq and --end_adapter_seq options. The default adapters are those for the Nanopore ligation adapters. To see what those sequences are and some alternatives, check out the adapter sequences page on the wiki. If you supply numbers for the adapter sequences (e.g. --start_adapter_seq 20, then Badread will make a random sequence of that length to be the adapter.
How much adapter is added to the start/end of a read is controlled by two parameters: rate and amount. These are set using the --start_adapter and --end_adapter options, with each taking two comma-delimited numbers (rate first and amount second). Rate is the percent chance that the adapter will appear at all. E.g. a start-adapter rate of 90 means that 10% of reads will have no adapter at their start and 90% of reads will have some adapter at their start. Think of it like a Bernoulli distribution.
Amount controls how much of the adapter, on average, appears on the read. E.g. a start-adapter amount of 60 means that when an adapter is on the start of the read, it has an expected length of 60% its full length. Start-adapters are truncated at the start and end-adapters are truncated at the end. The amount of adapter for each read is controlled by a beta distribution, and you can interactively explore different values using this Desmos plot.
To turn off adapters entirely, set the sequences to nothing:
--start_adapter_seq "" --end_adapter_seq ""
Badread can add two types of completely wrong reads to the output: junk and random. Junk reads are low-complexity sequence and simulate something wrong with the sequencer. A junk fragment might look like AATAATAATAATAATAATAAT (and so on). Random reads are made of random sequence (25% chance of each base at each position). By default, Badread includes 1% of each type, so 2% of the total reads will be junk or random.
Chimeric reads can occur in real datasets for two possible reasons: 1) fragments of DNA were actually ligated together before sequencing (probably more common in library preps that use ligase), and 2) two or more reads were sequenced in quick succession such that the sequencing software didn't recognise them as separate (an in silico chimera). Chimeras can occur on both PacBio and Nanopore sequencing platforms.
As an example, imagine you used --chimeras 2 to set chimeras to 2%. After making a sequence fragment, Badread then has a 2% chance of making another fragment and concatenating on to the first. It then has a 2% chance of concatenating on yet another fragment, and so on. This means that about 2% of the reads will be chimeras of two or more fragments, (2%)2 will be chimeras of three or more, (2%)3 will be chimeras of four or more, and so on. If you are using start/end adapters, they will sometimes (but not always) be added between the fragments.
Small circular plasmids can be underrepresented in long read sequencing – a topic addressed in Completing bacterial genome assemblies with multiplex MinION sequencing. In short, it is necessary to avoid excessive DNA shearing in order to achieve long read lengths. But (at least for ligation-based preps) an unsheared circular plasmid can't be sequenced because it has no blunt ends for adapter ligation. So plasmids on the low end of the read-length distribution will be sequenced at lower-than-expected depth.
Badread simulates this effect if you use the --small_plasmid_bias option. When turned on, Badread tosses out fragments that land in a circular reference which is smaller than the fragment size. The degree of bias is therefore strongly dependent on the fragment length distribution (specifically how much of the distribution is less than the plasmid's size). Note that this only affects circular sequences – linear sequences are unaffected.
Glitches are points in the read where the sequence is briefly messed up. They are controlled by three parameters:
- rate: how often glitches occur
- size: how much random sequence is added to the read
- skip: how much read sequence is lost
These are specified with the --glitches option by giving all three parameters in a comma-delimited list (no spaces). E.g. --glitches 5000,100,100. Each of these parameters is a mean for a geometric random variable. E.g. a glitch rate of 1000 doesn't mean glitches evenly occur at 1000 bp intervals, it means glitches are on average 1000 bp apart. Turn glitches off entirely with --glitches 0,0,0.
Take a look at the glitches page on the wiki to see some dotplots which illustrate the concept.
If you are interested in contributing to Badread, please take a look at the contribution guidelines.

