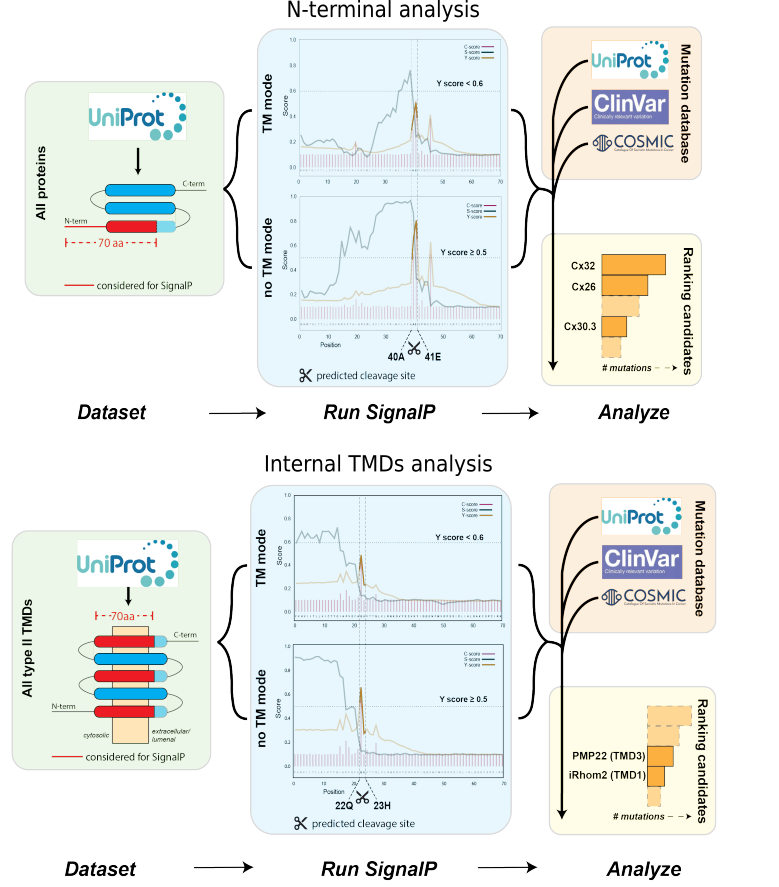
We performed a computational analysis on the first 70 aa at the N-t (N-terminal analysis) of the whole proteome and on 70 aa windows starting from all internal TMDs (Internal TMDs analysis) to identify potential SPC cleavage sites not associated with signal peptides (cryptic cleavage sites) by using a pre-existing a signal peptide prediction software SignalP and comparing the output of two networks (no-TM and TM modes). Our hits represent membrane protein with type-II oriented TMD predicted to be cleaved in no-TM network mode but not in TM network mode. Moreover, we rank the hits based on the number of disease-linked mutations.
Access the Shiny webApp here
We used the command-line version of the SignalP4.1 program. The program takes a proteins sequence (FASTA formatted) of 70 amino acids length as input and predicts the cleavage site and reports the Y-score (combined cleavage site score) in its output. To extract proteins with cryptic cleavage sites, we created two types of peptide sequences by considering the first 70 amino acids of all:
- proteins that lack a canonical signal peptide (N-terminal analysis)
- transmembrane domains of proteins that lack a canonical signal peptide (Internal TMDs analysis)
We predicted the Y-scores of both types of peptide sequences using the SignalP-TM (TM network mode) and SignalP-noTM (no-TM network mode) version (see Methods for details). A protein/transmembrane domain was considered to have a non-canonical cleavage site if its corresponding peptide’s Y-score in the SignalP-TM output was less than 0.6 and in SignalP-noTM output was more than 0.5. We retrieved all the proteins and their associated feature information from UniProt/Swiss-Prot. We assigned mutational information provided in (a) UniProt, COSMIC, and ClinVAR databases.
Validation of the hits (proteins predicted to have non-canonical cleavage sites) were then performed by ectopically expressing the WT (Wild Type) and mutant constructs in WT HEK293T cells or lacking the regulatory SPC subunit SPCS1. Cell lysates were then analyzed via Western blot to detect possible SPCS1-dependent cleavage fragments (see Methods for details).
| Location | Description |
|---|---|
| pyScripts/fetch_candidates.py | Script to extract candidates from UniProt/SwissProt's human.dat.gz file and run modified SignalP4.1. |
| pyScripts/plot_scatter.py | Script to generate and plot the data. |
| data/part_a_fasta | directory with peptide FASTA sequences given as input to SignalP. |
| data/part_a_signalp | output of SignalP peptide sequence prediction. |
| data/part_a_data.tsv | output of SignalP and protein information in TSV format. |
| Location | Description |
|---|---|
| pyScripts/fetch_candidates_part_C.py | Script to extract candidates from UniProt/SwissProt's human.dat.gz file and run modified SignalP4.1. |
| pyScripts/plot_scatter_part_C.py | Script to generate and plot the data. |
| data/part_c_annotations.tsv | Annotations of TM domains in proteins. |
| data/part_c_fasta | directory with peptide FASTA sequences given as input to SignalP. |
| data/part_c_signalp | output of SignalP peptide sequence prediction. |
| data/part_c_data.tsv | output of SignalP and protein information in TSV format. |
| Location | Description |
|---|---|
| signalp-4.1/signalp | Original signalp-4.1 script. |
| signalp-4.1/signalp_TM | Customized signalp-4.1 script in which the TM_TRESHOLD is set to -1 so that it is always less than TMCount and thus forces the program to run in the TM network mode. |
| signalp-4.1/signalp_noTM | Customized signalp-4.1 script in which the TM_TRESHOLD is set to 100 so that it is always greater than TMCount and thus forces the program to run in the no-TM network mode. |
Note: The information about the data downloaded from UniProt, COSMIC and ClinVar can be retrieved from Materials and Methods of the accompanying manuscript.
| Location | Description |
|---|---|
| data/server.R | Script to run the Shiny webApp. |
| data/ui.R | Script to run the Shiny webApp. |
| data/workflow* | Workflow figure shown in the REAMDE. |
Marius Lemberg: m.lemberg@uni-koeln.de (Lemberg lab, Heidelberg/Cologne)
Matthias Feige: matthias.feige@tum.de (CPB lab, Munich)
Gurdeep Singh: gurdeep.singh@bioquant.uni-heidelberg.de (Russell lab, Heidelberg)
Zanotti A, Coelho JPL, Kaylani D, Singh G, Tauber M, Hitzenberger M, Avci D, Zacharias M, Russell RB, Lemberg MK, Feige MJ The human signal peptidase complex acts as a quality control enzyme for membrane proteins. Science (2022). doi: 10.1126/science.abo5672 PMID: 36454823