Deep generative models are rapidly becoming popular for the discovery of new molecules and materials. Such models learn on a large collection of molecular structures and produce novel compounds. In this work, we introduce Molecular Sets (MOSES), a benchmarking platform to support research on machine learning for drug discovery. MOSES implements several popular molecular generation models and provides a set of metrics to evaluate the quality and diversity of generated molecules. With MOSES, we aim to standardize the research on molecular generation and facilitate the sharing and comparison of new models.
For more details, please refer to the paper.
If you are using MOSES in your research paper, please cite us as
@article{10.3389/fphar.2020.565644,
title={{M}olecular {S}ets ({MOSES}): {A} {B}enchmarking {P}latform for {M}olecular {G}eneration {M}odels},
author={Polykovskiy, Daniil and Zhebrak, Alexander and Sanchez-Lengeling, Benjamin and Golovanov, Sergey and Tatanov, Oktai and Belyaev, Stanislav and Kurbanov, Rauf and Artamonov, Aleksey and Aladinskiy, Vladimir and Veselov, Mark and Kadurin, Artur and Johansson, Simon and Chen, Hongming and Nikolenko, Sergey and Aspuru-Guzik, Alan and Zhavoronkov, Alex},
journal={Frontiers in Pharmacology},
year={2020}
}
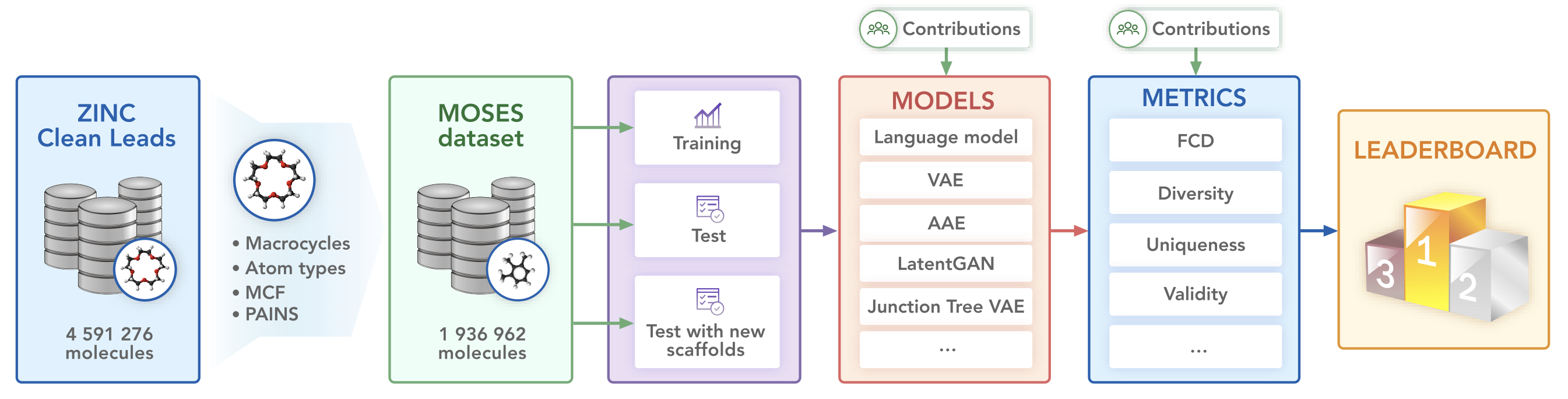
We propose a benchmarking dataset refined from the ZINC database.
The set is based on the ZINC Clean Leads collection. It contains 4,591,276 molecules in total, filtered by molecular weight in the range from 250 to 350 Daltons, a number of rotatable bonds not greater than 7, and XlogP less than or equal to 3.5. We removed molecules containing charged atoms or atoms besides C, N, S, O, F, Cl, Br, H or cycles longer than 8 atoms. The molecules were filtered via medicinal chemistry filters (MCFs) and PAINS filters.
The dataset contains 1,936,962 molecular structures. For experiments, we split the dataset into a training, test and scaffold test sets containing around 1.6M, 176k, and 176k molecules respectively. The scaffold test set contains unique Bemis-Murcko scaffolds that were not present in the training and test sets. We use this set to assess how well the model can generate previously unobserved scaffolds.
- Character-level Recurrent Neural Network (CharRNN)
- Variational Autoencoder (VAE)
- Adversarial Autoencoder (AAE)
- Junction Tree Variational Autoencoder (JTN-VAE)
- Latent Generative Adversarial Network (LatentGAN)
Besides standard uniqueness and validity metrics, MOSES provides other metrics to access the overall quality of generated molecules. Fragment similarity (Frag) and Scaffold similarity (Scaff) are cosine distances between vectors of fragment or scaffold frequencies correspondingly of the generated and test sets. Nearest neighbor similarity (SNN) is the average similarity of generated molecules to the nearest molecule from the test set. Internal diversity (IntDiv) is an average pairwise similarity of generated molecules. Fréchet ChemNet Distance (FCD) measures the difference in distributions of last layer activations of ChemNet. Novelty is a fraction of unique valid generated molecules not present in the training set.
| Model | Valid (↑) | Unique@1k (↑) | Unique@10k (↑) | FCD (↓) | SNN (↑) | Frag (↑) | Scaf (↑) | IntDiv (↑) | IntDiv2 (↑) | Filters (↑) | Novelty (↑) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test | TestSF | Test | TestSF | Test | TestSF | Test | TestSF | ||||||||
| Train | 1.0 | 1.0 | 1.0 | 0.008 | 0.4755 | 0.6419 | 0.5859 | 1.0 | 0.9986 | 0.9907 | 0.0 | 0.8567 | 0.8508 | 1.0 | 1.0 |
| HMM | 0.076±0.0322 | 0.623±0.1224 | 0.5671±0.1424 | 24.4661±2.5251 | 25.4312±2.5599 | 0.3876±0.0107 | 0.3795±0.0107 | 0.5754±0.1224 | 0.5681±0.1218 | 0.2065±0.0481 | 0.049±0.018 | 0.8466±0.0403 | 0.8104±0.0507 | 0.9024±0.0489 | 0.9994±0.001 |
| NGram | 0.2376±0.0025 | 0.974±0.0108 | 0.9217±0.0019 | 5.5069±0.1027 | 6.2306±0.0966 | 0.5209±0.001 | 0.4997±0.0005 | 0.9846±0.0012 | 0.9815±0.0012 | 0.5302±0.0163 | 0.0977±0.0142 | 0.8738±0.0002 | 0.8644±0.0002 | 0.9582±0.001 | 0.9694±0.001 |
| Combinatorial | 1.0±0.0 | 0.9983±0.0015 | 0.9909±0.0009 | 4.2375±0.037 | 4.5113±0.0274 | 0.4514±0.0003 | 0.4388±0.0002 | 0.9912±0.0004 | 0.9904±0.0003 | 0.4445±0.0056 | 0.0865±0.0027 | 0.8732±0.0002 | 0.8666±0.0002 | 0.9557±0.0018 | 0.9878±0.0008 |
| CharRNN | 0.9748±0.0264 | 1.0±0.0 | 0.9994±0.0003 | 0.0732±0.0247 | 0.5204±0.0379 | 0.6015±0.0206 | 0.5649±0.0142 | 0.9998±0.0002 | 0.9983±0.0003 | 0.9242±0.0058 | 0.1101±0.0081 | 0.8562±0.0005 | 0.8503±0.0005 | 0.9943±0.0034 | 0.8419±0.0509 |
| AAE | 0.9368±0.0341 | 1.0±0.0 | 0.9973±0.002 | 0.5555±0.2033 | 1.0572±0.2375 | 0.6081±0.0043 | 0.5677±0.0045 | 0.991±0.0051 | 0.9905±0.0039 | 0.9022±0.0375 | 0.0789±0.009 | 0.8557±0.0031 | 0.8499±0.003 | 0.996±0.0006 | 0.7931±0.0285 |
| VAE | 0.9767±0.0012 | 1.0±0.0 | 0.9984±0.0005 | 0.099±0.0125 | 0.567±0.0338 | 0.6257±0.0005 | 0.5783±0.0008 | 0.9994±0.0001 | 0.9984±0.0003 | 0.9386±0.0021 | 0.0588±0.0095 | 0.8558±0.0004 | 0.8498±0.0004 | 0.997±0.0002 | 0.6949±0.0069 |
| JTN-VAE | 1.0±0.0 | 1.0±0.0 | 0.9996±0.0003 | 0.3954±0.0234 | 0.9382±0.0531 | 0.5477±0.0076 | 0.5194±0.007 | 0.9965±0.0003 | 0.9947±0.0002 | 0.8964±0.0039 | 0.1009±0.0105 | 0.8551±0.0034 | 0.8493±0.0035 | 0.976±0.0016 | 0.9143±0.0058 |
| LatentGAN | 0.8966±0.0029 | 1.0±0.0 | 0.9968±0.0002 | 0.2968±0.0087 | 0.8281±0.0117 | 0.5371±0.0004 | 0.5132±0.0002 | 0.9986±0.0004 | 0.9972±0.0007 | 0.8867±0.0009 | 0.1072±0.0098 | 0.8565±0.0007 | 0.8505±0.0006 | 0.9735±0.0006 | 0.9498±0.0006 |
For comparison of molecular properties, we computed the Wasserstein-1 distance between distributions of molecules in the generated and test sets. Below, we provide plots for lipophilicity (logP), Synthetic Accessibility (SA), Quantitative Estimation of Drug-likeness (QED) and molecular weight.
| logP | SA |
|---|---|
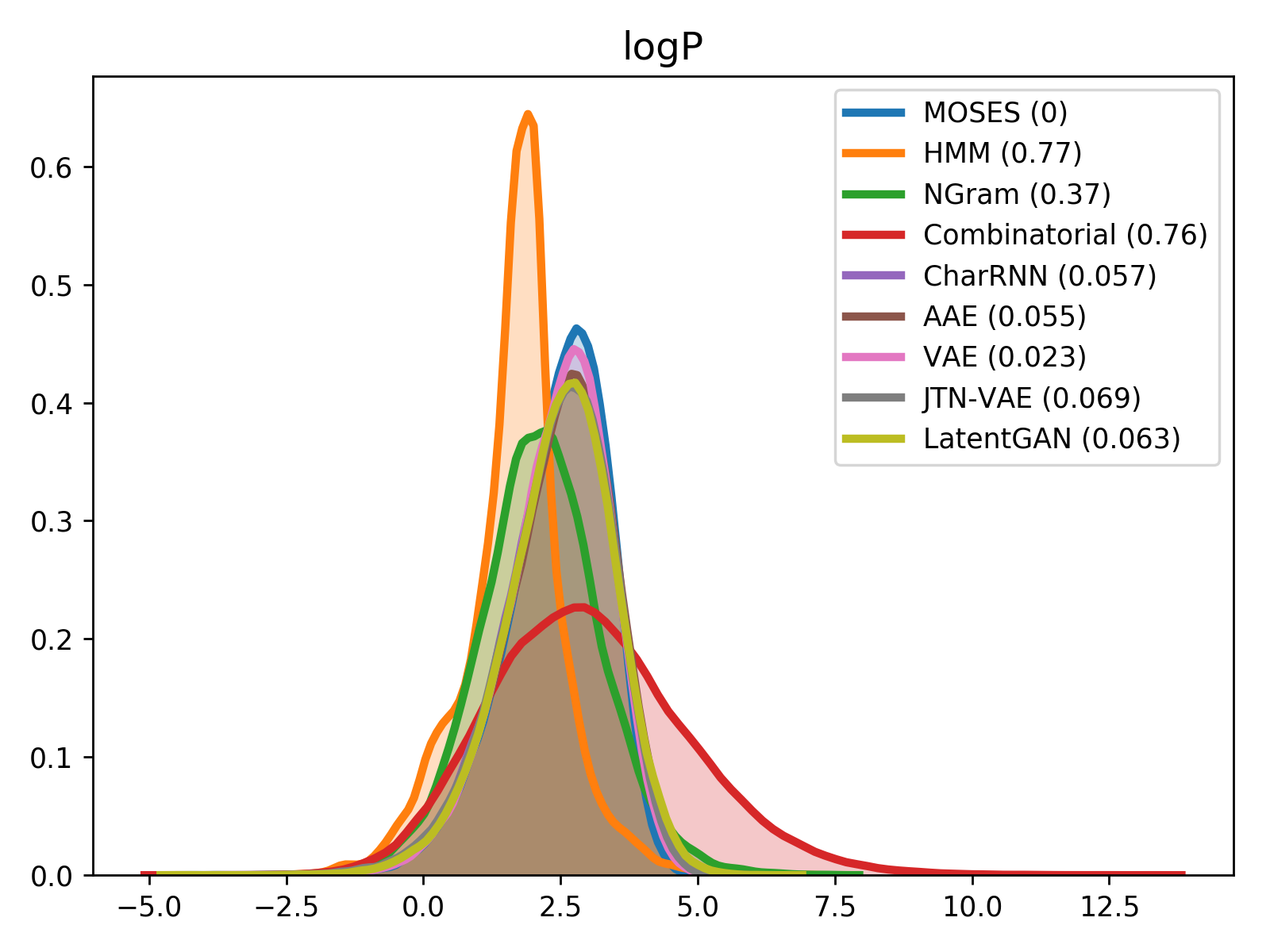 |
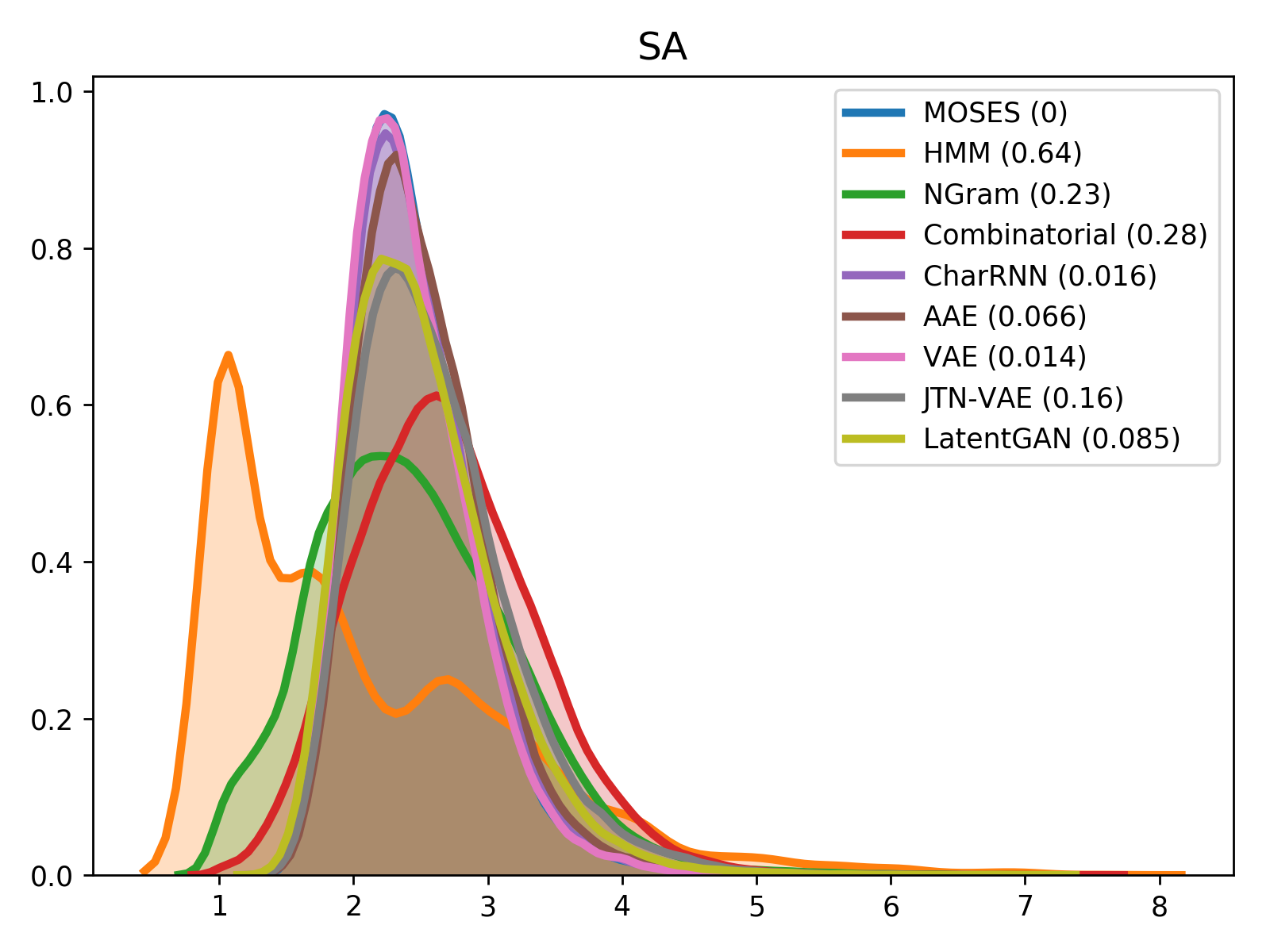 |
| weight | QED |
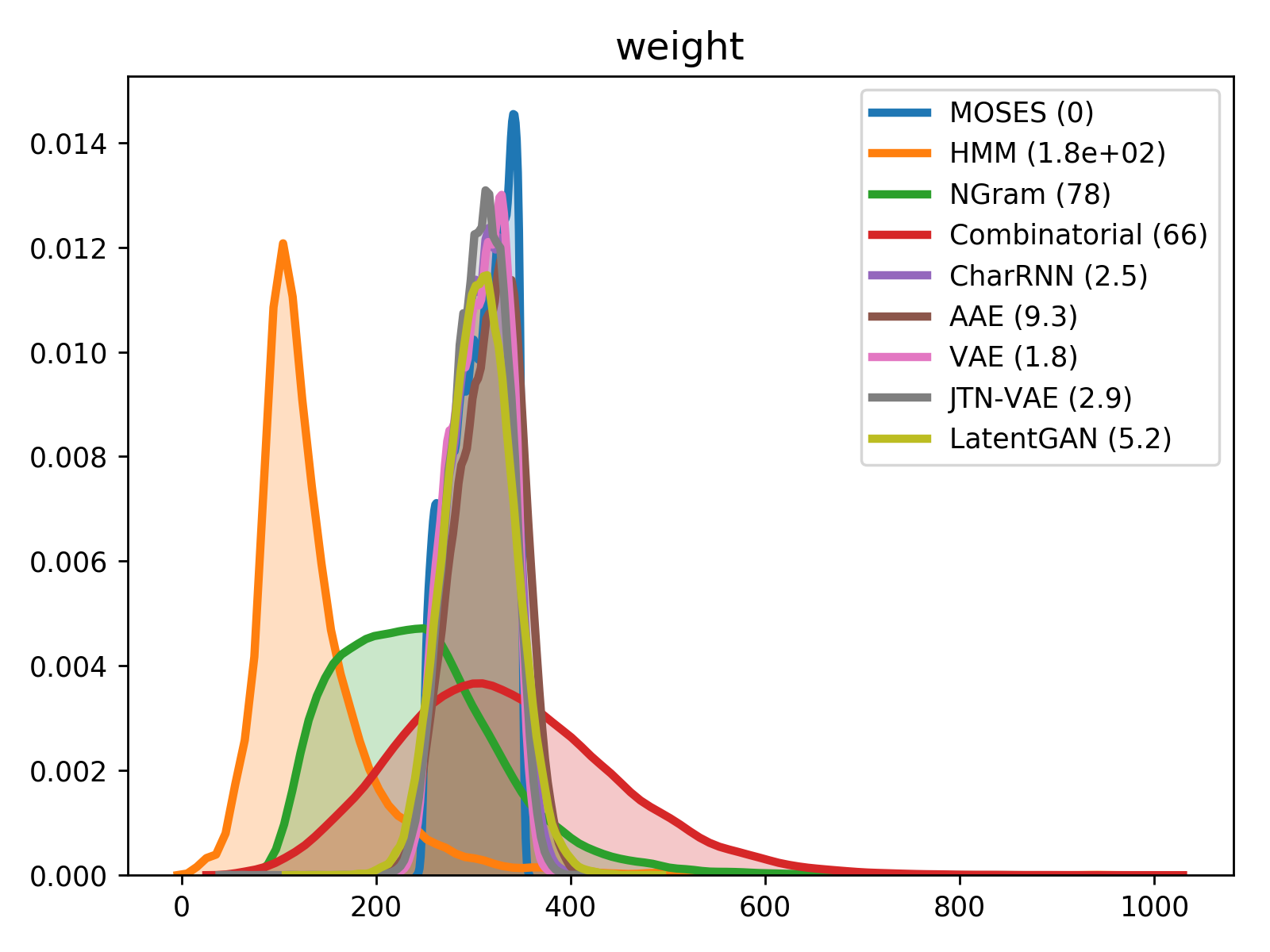 |
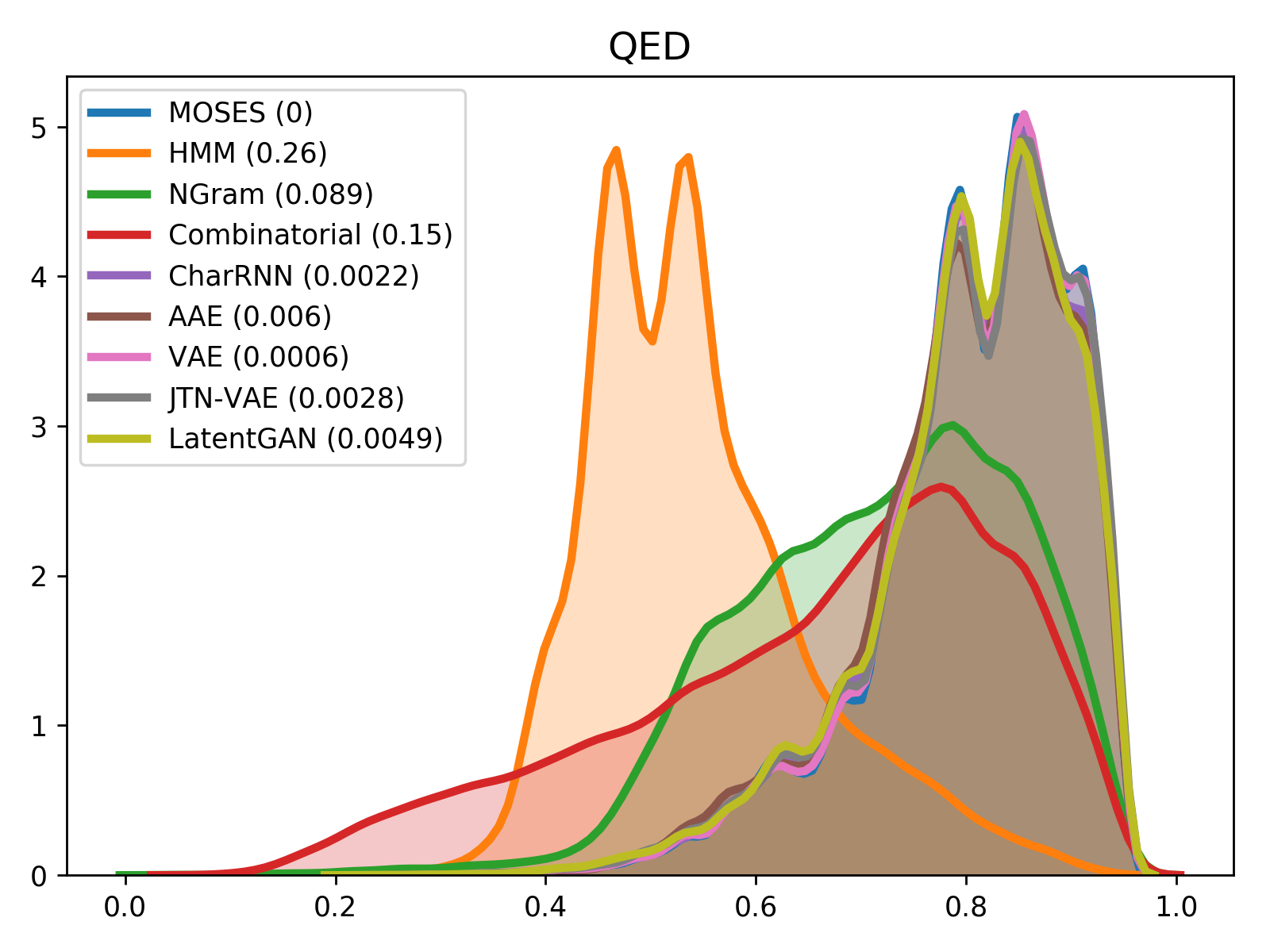 |
The simplest way to install MOSES (models and metrics) is to install RDKit: conda install -yq -c rdkit rdkit and then install MOSES (molsets) from pip (pip install molsets). If you want to use LatentGAN, you should also install additional dependencies using bash install_latentgan_dependencies.sh.
If you are using Ubuntu, you should also install sudo apt-get install libxrender1 libxext6 for RDKit.
-
Install docker and nvidia-docker.
-
Pull an existing image (4.1Gb to download) from DockerHub:
docker pull molecularsets/mosesor clone the repository and build it manually:
git clone https://github.com/molecularsets/moses.git
nvidia-docker image build --tag molecularsets/moses moses/- Create a container:
nvidia-docker run -it --name moses --network="host" --shm-size 10G molecularsets/moses- The dataset and source code are available inside the docker container at /moses:
docker exec -it molecularsets/moses bashAlternatively, install dependencies and MOSES manually.
- Clone the repository:
git lfs install
git clone https://github.com/molecularsets/moses.git-
Install RDKit for metrics calculation.
-
Install MOSES:
python setup.py install- (Optional) Install dependencies for LatentGAN:
bash install_latentgan_dependencies.sh-
Install MOSES as described in the previous section.
-
Get
train,testandtest_scaffoldsdatasets using the following code:
import moses
train = moses.get_dataset('train')
test = moses.get_dataset('test')
test_scaffolds = moses.get_dataset('test_scaffolds')- You can use a standard torch DataLoader in your models. We provide a simple
StringDatasetclass for convenience:
from torch.utils.data import DataLoader
from moses import CharVocab, StringDataset
train = moses.get_dataset('train')
vocab = CharVocab.from_data(train)
train_dataset = StringDataset(vocab, train)
train_dataloader = DataLoader(
train_dataset, batch_size=512,
shuffle=True, collate_fn=train_dataset.default_collate
)
for with_bos, with_eos, lengths in train_dataloader:
...- Calculate metrics from your model's samples. We recomend sampling at least
30,000molecules:
import moses
metrics = moses.get_all_metrics(list_of_generated_smiles)- Add generated samples and metrics to your repository. Run the experiment multiple times to estimate the variance of the metrics.
You can run pretty much everything with:
python scripts/run.pyThis will split the dataset, train the models, generate new molecules, and calculate the metrics. Evaluation results will be saved in metrics.csv.
You can specify the GPU device index as cuda:n (or cpu for CPU) and/or model by running:
python scripts/run.py --device cuda:1 --model aaeFor more details run python scripts/run.py --help.
You can reproduce evaluation of all models with several seeds by running:
sh scripts/run_all_models.shpython scripts/train.py <model name> \
--train_load <train dataset> \
--model_save <path to model> \
--config_save <path to config> \
--vocab_save <path to vocabulary>To get a list of supported models run python scripts/train.py --help.
For more details of certain model run python scripts/train.py <model name> --help.
python scripts/sample.py <model name> \
--model_load <path to model> \
--vocab_load <path to vocabulary> \
--config_load <path to config> \
--n_samples <number of samples> \
--gen_save <path to generated dataset>To get a list of supported models run python scripts/sample.py --help.
For more details of certain model run python scripts/sample.py <model name> --help.
python scripts/eval.py \
--ref_path <reference dataset> \
--gen_path <generated dataset>For more details run python scripts/eval.py --help.