An R Package for the analysis of RNA editing data from studies utilizing whole genome sequencing and RNA-seq from the same individual(s).
editTools was made out of the necessity to create a reproducible means to analyze RNA editing data. Candidate RNA editing detection is most often performed using scripts to analyze variant call format (VCF) data, however such scripts are rarely published leading to the possibility that variation in RNA editing results may be partly due to subtle differences in these scripts are designed. This R package provides the means to analyze VCF data using standard methodology inspired from previous studies, and do so within the R framework where powerful graphical tools can be utilized to best explore the data.
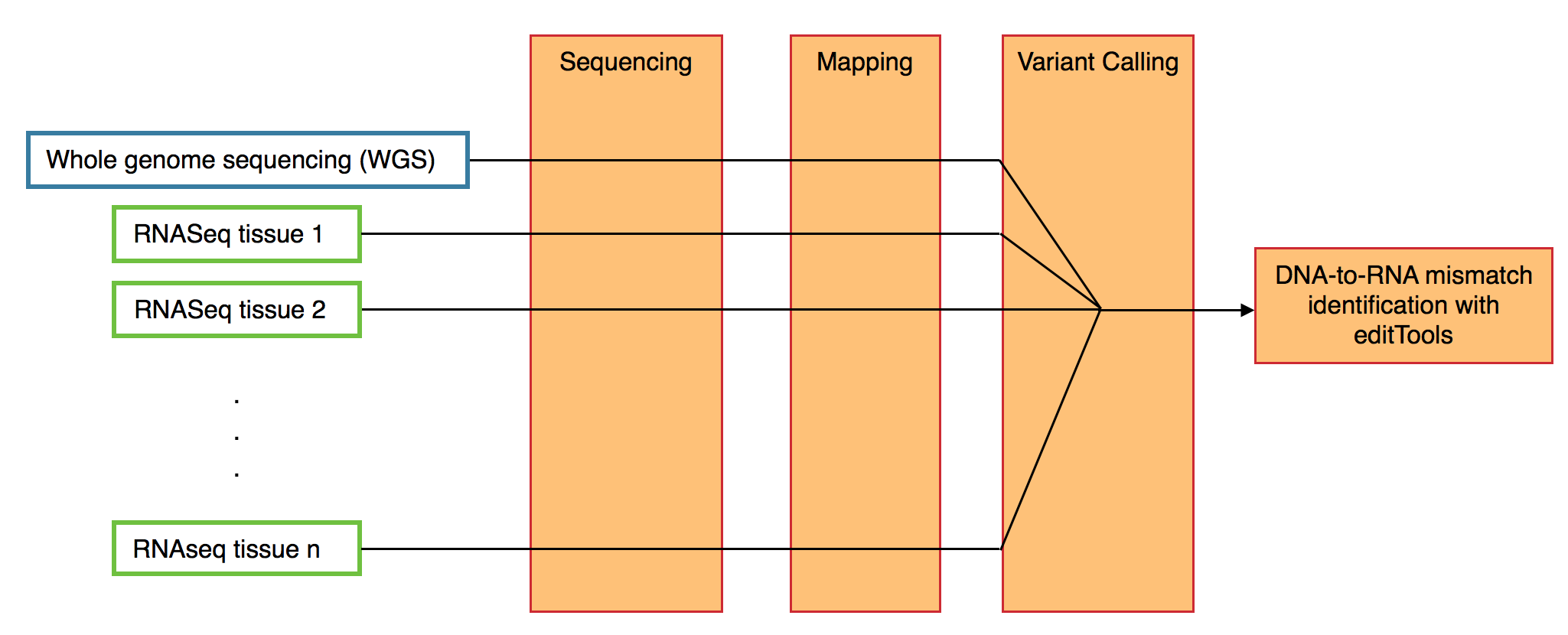
From a single individual, editTools can analyze Whole genome sequencing (WGS) as well as RNAseq from any number of tissues. Input for editTools can be prepared a number of ways (allowing for differential sequencing, mapping, and variant calling techniques).
An example of how I prepare input for editTools can be found here
editTools contains compiled code and relies on the Rcpp package and C++11.
If using GNU version 4.7 or later, specify that you want to install editTools using C++11 with:
Sys.setenv("PKG_CXXFLAGS" = "-std=c++11")Otherwise, use:
Sys.setenv("PKG_CXXFLAGS" = "-std=c++0x")Then install the editTools package with:
devtools::install_github("funkhou9/editTools")The only current requirement for editTools is that the input (resulting from the Variant Calling software of choice), must be in VCF format and contain the following header information.
#CHROM POS ID REF ALT QUAL FILTER INFO FORMAT <DNA> <RNA1> <RNA2> <RNA3> ...
where <DNA> corresponds to your WGS sample, and <RNA1>, <RNA2>, <RNA3> ... correspond to any number of RNA samples. Currently, the INFO field must contain DP and DV tags to represent total sequencing depth and variant depth, respectively.
VCF format is (currently, to my knowledge) unaware of strand specificity. In other words, all bases reported in a VCF file correspond to the TOP strand of the genome. This presents a challenge when calling transcriptome variants. For RNA editing discovery, this means that one cannot distinguish an A-to-G (DNA-to-RNA) mismatch from a T-to-C mismatch, since a single cDNA read will contain both the G allele and the C allele, and knowledge of which strand that cDNA read was generated from is absent from VCF files. To workaround this and have the ability to distinguish (for example) A-to-G mismatches from T-to-C mismatches, the user can prepare two VCF files, one containing variants on plus-strand transcripts for each <RNA> sample, and one containing variants on minus-strand transcripts for each <RNA> sample. In each case, the same <DNA> sample is provided.
If two VCF files are prepared (see above), then identification of DNA-to-RNA mismatches can be performed with:
edits <- editTools::find_edits(<plus.vcf>, <minus.vcf>, names = c("DNA", "Brain", "Liver", "Heart"))<plus.vcf>VCF file containing plus-strand transcripts for each RNA sample (a character string).<minus.vcf>VCF file containing minus-strand transcripts for each RNA sample (a character string).namesDesired names for DNA and RNA samples (a character vector).
For information on all advanced options to tweak mismatch idenfication parameters, see:
?editTools::find_editsedits is an object of class "edit_table", and initially contains two fields: AllSites and Tissues.
-
Allsitesis a data.frame with candidate RNA editing events in the rows with the following columns:IDA numerical ID associated with the tissue specific candidate editing event.ChrA character representing the chromosome of the edit.PosA numeric representing the chromosomal position of the edit.StrandA character representing the strand of the edited transcript ("+" or "-")MismatchA character representing the mismatch (eg. "AtoG")DNA_depthA numeric representing the total DNA sample sequencing depthDNA_variant_depthA numeric representing the DNA sample sequencing depth that is in support of a variantRNA_depthA numeric representing the total RNA sample sequencing depthRNA_mismatch_depthA numeric representing the RNA sample sequencing depth in support of a DNA-to-RNA mismatchRNA_edit_fracA numeric. Simply theRNA_mismatch_depth/RNA_depthPhred_strand_biasA numeric representing the phred-scaled probabilities of strand-bias.Ave_MQA numeric representing the average mapping quality at this site across all samples.TissueA character indicating which tissue (named withnamesargument) the event was found in.
-
Tissuesis a list with the number of elements equal to the number of tissues studied. Each element is a data.frame with mismatch types (A-to-G) in rows and the following columns:MismatchA character with the type of mismatchFreqThe number of events found of the specified type of mismatchPropThe proportion of mismatches belonging to the specified type
- Update this documentation with more examples on how to use
edit_tableobjects. - Monumental changes to program design - Incorporate new input types. The ability to process BAM files instead of VCF files could greatly enhance editTools ease of use. For instance, separating plus-strand alignments from minus-strand alignments would no longer be needed by the user.