This benchmark is based on the publicly available long-read sequencing data (i.e., Oxford Nanopore PromethION long-reads) of the Ashkenazim son HG002/NA24385. I provide each step how to reproduce the final metrics with publicly available tools.
Thanks for armintoepfer's great works!
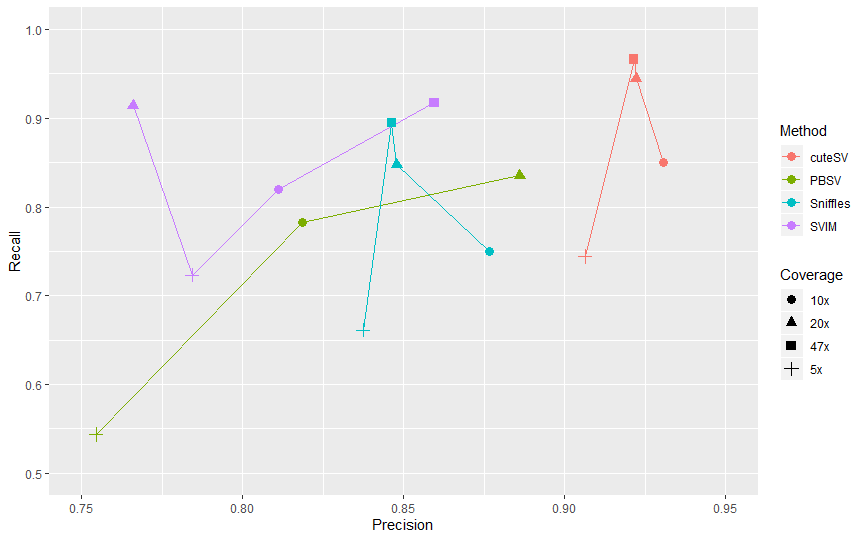
Fig 1. Recall & Precision
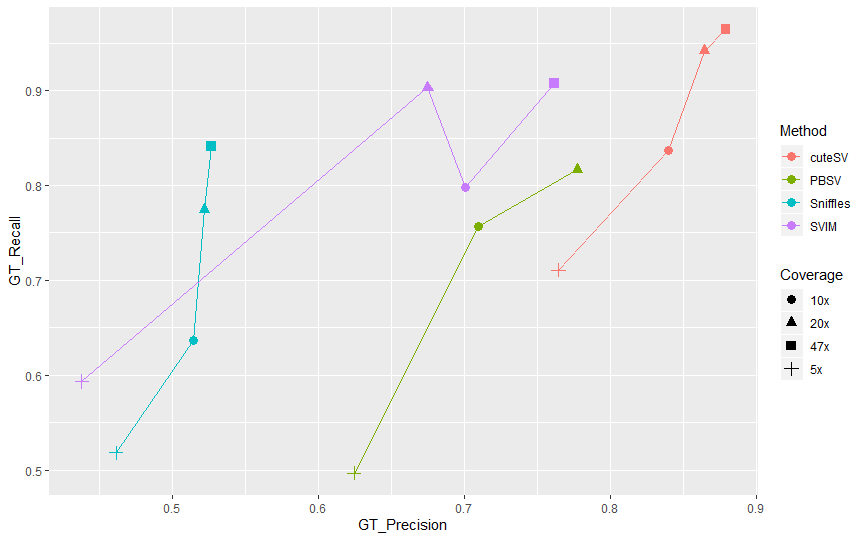
Fig 2. GT-Recall & GT-Precision
| Method | F1 % | Precision % | Recall % | GT-F1 % | GT-Precision % | GT-Recall % |
|---|---|---|---|---|---|---|
| pbsv | 63.20 | 75.45 | 54.37 | 55.33 | 62.46 | 49.66 |
| svim | 75.25 | 78.43 | 72.33 | 50.40 | 43.80 | 59.34 |
| sniffles | 73.89 | 83.74 | 66.11 | 48.82 | 46.15 | 51.81 |
| cuteSV | 81.74 | 90.63 | 74.44 | 73.66 | 76.45 | 71.07 |
| Method | F1 % | Precision % | Recall % | GT-F1 % | GT-Precision % | GT-Recall % |
|---|---|---|---|---|---|---|
| pbsv | 79.99 | 81.86 | 78.20 | 73.23 | 70.96 | 75.66 |
| svim | 81.55 | 81.11 | 81.99 | 74.60 | 70.09 | 79.74 |
| sniffles | 80.80 | 87.66 | 74.94 | 56.90 | 51.42 | 63.69 |
| cuteSV | 88.85 | 93.07 | 85.00 | 83.83 | 84.00 | 83.65 |
| Method | F1 % | Precision % | Recall % | GT-F1 % | GT-Precision % | GT-Recall % |
|---|---|---|---|---|---|---|
| pbsv | 85.98 | 88.60 | 83.52 | 79.65 | 77.75 | 81.64 |
| svim | 83.35 | 76.60 | 91.39 | 77.26 | 67.49 | 90.34 |
| sniffles | 84.78 | 84.78 | 84.78 | 62.37 | 52.21 | 77.43 |
| cuteSV | 93.34 | 92.22 | 94.49 | 90.13 | 86.44 | 94.15 |
| Method | F1 % | Precision % | Recall % | GT-F1 % | GT-Precision % | GT-Recall % |
|---|---|---|---|---|---|---|
| pbsv | -- | -- | -- | -- | -- | -- |
| svim | 88.74 | 85.95 | 91.72 | 82.82 | 76.16 | 90.76 |
| sniffles | 86.98 | 84.63 | 89.46 | 64.78 | 52.69 | 84.09 |
| cuteSV | 94.33 | 92.15 | 96.61 | 91.98 | 87.91 | 96.45 |
Information how to install conda and add the bioconda channel is available
on https://bioconda.github.io/.
conda create --name sv python=3
source activate sv
conda install minimap2==2.17 pbsv==2.2.0 svim==1.2.0 sniffles==1.0.11 cuteSV==1.0.3 truvari==1.2 samtools bgzip tabix- Create directory structure:
mkdir -p fastqs ref alns tools/{pbsv,sniffles,svim,cuteSV} giab- Download genome in a bottle annotations:
FTPDIR=ftp://ftp-trace.ncbi.nlm.nih.gov/giab/ftp/data/AshkenazimTrio/analysis/
curl -s ${FTPDIR}/NIST_SVs_Integration_v0.6/HG002_SVs_Tier1_v0.6.bed > giab/HG002_SVs_Tier1_v0.6.bed
curl -s ${FTPDIR}/NIST_SVs_Integration_v0.6/HG002_SVs_Tier1_v0.6.vcf.gz > giab/HG002_SVs_Tier1_v0.6.vcf.gz- Download hg19 reference with decoys and map non-ACGT characters to N:
curl -s ftp://ftp-trace.ncbi.nih.gov/1000genomes/ftp/technical/reference/phase2_reference_assembly_sequence/hs37d5.fa.gz > ref/human_hs37d5.fasta.gz
gunzip ref/human_hs37d5.fasta.gz
sed -i '/^[^>]/ y/BDEFHIJKLMNOPQRSUVWXYZbdefhijklmnopqrsuvwxyz/NNNNNNNNNNNNNNNNNNNNNNNNNNNNNNNNNNNNNNNNNNNN/' ref/human_hs37d5.fasta- Download hg19 tandem repeat annotations:
curl -s https://raw.githubusercontent.com/PacificBiosciences/pbsv/master/annotations/human_hs37d5.trf.bed > ref/human_hs37d5.trf.bed- Download all
.fastqfiles:
FTPDIR=ftp://ftp.ncbi.nlm.nih.gov/giab/ftp/data/AshkenazimTrio/HG002_NA24385_son/UCSC_Ultralong_OxfordNanopore_Promethion/
for fastq in $(curl -s -l ${FTPDIR} | grep '.fastq'); do curl -s ${FTPDIR}${fastq} > fastqs/${fastq}; done
for fastq in `ls fastqs`; do gunzip fastqs/${fastq}; done- Align each movie:
for i in `ls fastqs/`;do
minimap2 ref/human_hs37d5.fasta fastq/$i -a -z 600,200 -x map-ont \
--MD -Y -o alns/$i.sam -R '@RG\tID:hg2'
done- Alignments sorting and merging:
for i in `ls alns/`;do
samtools view -buS alns/$i | samtools sort -O bam -T ./ - > alns/$i.bam
done
for i in `ls alns/*.bam`; do echo $i; done > input_bam.fofn
samtools merge alns/GM24385_all.bam -b input_bam.fofn && samtools index alns/GM24385_all.bam8a) Discover SV signatures for each alignment, can be done in parallel:
pbsv discover alns/GM24385_all.bam tools/pbsv/hg2_ont.svsig.gz" --tandem-repeats ref/human_hs37d5.trf.bed8b) Call and polish SVs:
pbsv call ref/human_hs37d5.fasta tools/pbsv/hg2_ont.svsig.gz tools/pbsv/hg2_ont.pbsv.vcf -t INS,DEL
bgzip tools/pbsv/hg2_ont.pbsv.vcf
tabix tools/pbsv/hg2_ont.pbsv.vcf.gz9a) Run svim:
svim alignment tools/svim alns/GM24385_all.bam ref/human_hs37d5.fasta --min_sv_size 309b) Prepare for truvari:
cat tools/svim/final_results.vcf \
| sed 's/INS:NOVEL/INS/g' \
| sed 's/DUP:INT/INS/g' \
| sed 's/DUP:TANDEM/INS/g' \
| awk '{ if($1 ~ /^#/) { print $0 } else { if($5=="<DEL>" || $5=="<INS>") { print $0 }}}' \
| grep -v 'SUPPORT=1;\|SUPPORT=2;\|SUPPORT=3;\|SUPPORT=4;\|SUPPORT=5;\|SUPPORT=6;\|SUPPORT=7;\|SUPPORT=8;\|SUPPORT=9;' \
| sed 's/q5/PASS/g' > tools/svim/hg2_ont.svim.vcf
bgzip tools/svim/hg2_ont.svim.vcf
tabix tools/svim/hg2_ont.svim.vcf.gz10a) Run sniffles:
sniffles -s 10 -l 30 -m alns/GM24385_all.bam -v tools/sniffles/hg2_ont.sniffles.vcf --genotype10b) Prepare for truvari:
cat <(cat tools/sniffles/hg2_ont.sniffles.vcf | grep "^#") \
<(cat tools/sniffles/hg2_ont.sniffles.vcf | grep -vE "^#" | \
grep 'DUP\|INS\|DEL' | sed 's/DUP/INS/g' | sort -k1,1 -k2,2g) \
| bgzip -c > tools/sniffles/hg2_ont.sniffles.vcf.gz
tabix tools/sniffles/hg2_ont.sniffles.vcf.gz11a) Run cuteSV:
cuteSV alns/GM24385_all.bam tools/cuteSV/hg2_ont.cuteSV.vcf ./ -s 10 -l 3011b) Prepare for truvari:
grep -v 'INV\|DUP\|BND' tools/cuteSV/hg2_ont.cuteSV.vcf | bgzip -c > tools/cuteSV/hg2_ont.cuteSV.vcf.gz
tabix tools/cuteSV/hg2_ont.cuteSV.vcf.gz- Compare to ground truth:
truvari -f ref/human_hs37d5.fasta -b giab/HG002_SVs_Tier1_v0.6.vcf.gz\
--includebed giab/HG002_SVs_Tier1_v0.6.bed -o bench-pbsv --passonly\
--giabreport -r 1000 -p 0.00 -c tools/pbsv/hg2.pbsv.vcf.gz
truvari -f ref/human_hs37d5.fasta -b giab/HG002_SVs_Tier1_v0.6.vcf.gz\
--includebed giab/HG002_SVs_Tier1_v0.6.bed -o bench-svim --passonly\
--giabreport -r 1000 -p 0.00 -c tools/svim/hg2.svim.vcf.gz
truvari -f ref/human_hs37d5.fasta -b giab/HG002_SVs_Tier1_v0.6.vcf.gz\
--includebed giab/HG002_SVs_Tier1_v0.6.bed -o bench-sniffles --passonly\
--giabreport -r 1000 -p 0.00 -c tools/sniffles/hg2.sniffles.vcf.gz
truvari -f ref/human_hs37d5.fasta -b giab/HG002_SVs_Tier1_v0.6.vcf.gz\
--includebed giab/HG002_SVs_Tier1_v0.6.bed -o bench-cuteSV --passonly\
--giabreport -r 1000 -p 0.00 -c tools/cuteSV/hg2_ont.cuteSV.vcf.gz- Parse results:
function sumsv() { cat $1 | grep ':' | tr -d ',' |sed "s/^[ \t]*//"| tr -d '"' |\
tr -d ' ' | tr ':' '\t' | awk '{ for (i=1; i<=NF; i++) {
a[NR,i] = $i } } NF>p { p = NF } END { for(j=1; j<=p; j++)
{ str=a[1,j]; for(i=2; i<=NR; i++){ str=str" "a[i,j]; } print str } }' |\
tail -n 1 | awk '{ printf "%1.4f\t%1.4f\t%1.4f\t%10.0f\t%10.0f\t%10.0f\n", $2,$4,$11,$1,$8,$1+$8 }';}
cat <(echo -e "Run\tF1\tPrecision\tRecall\tFP\tFN\tFP+FN")\
<(cat <(for i in bench*; do printf $i"\t";sumsv $i/summary.txt;done) |\
sed 's/bench//g;s/-//g' | sort -k 2 -n -r) | column -tOr for markdown:
cat <(echo -e "Run\tF1\tPrecision\tRecall\tFP\tFN\tFP+FN")\
<(echo -e ":-:\t:-:\t:-:\t:-:\t:-:\t:-:\t:-:")\
<(cat <(for i in bench*; do printf $i"\t";sumsv $i/summary.txt;done) |\
sed 's/bench//g;s/-//g' | sort -k 2 -n -r) | tr '\t' ' ' | tr -s ' ' | tr ' ' '|' | awk '{print "|"$0"|"}'- Create a new base folder for each coverage and subsample the merged BAM:
samtools view -bS -s 0.106 alns/GM24385_all.bam > alns/GM24385_all_5x.bam
samtools view -bS -s 0.213 alns/GM24385_all.bam > alns/GM24385_all_10x.bam
samtools view -bS -s 0.426 alns/GM24385_all.bam > alns/GM24385_all_20x.bamRepeat steps to run pbsv, sniffles, svim and cuteSV.
For sniffles, svim and cuteSV, I respectively uesd support-read 2, 3, 4 and 10 for 5x, 10x, 20x and 47x.