#HiCPlotter: Integrating HiC data with genomic datasets
HiCPlotter is a Python data visualization tool for integrating different data types with interaction matrixes. For more on 5C or HiC, please check: Dekker et al. 2013.
HiCPlotter is designed by Kadir Caner Akdemir (kcakedemir at mdanderson dot org / find me on Twitter) in Lynda Chin's Lab at the University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Python 2.7.*
- Please note: scipy, numpy and matplotlib modules should be installed and updated to current version. Following versions of numpy (1.9.0, 1.9.2), scipy(0.14.0, 0.15.1) and matplotlib(1.3.1, 1.4.3) have been tested successfully.
- If you receive error(s) related to one or more of these modules, check this solution and/or check versions of python and required modules.
- If you receive an error about jpeg encoder, please check Tips section below.
HiCPlotter is tested on Mac OS (Mountain Lion and Yosemite) and Linux (RedHat 4.1.2-44 and 5.5-Final) systems.
HiCPlotter is purposefully designed with the least amount of dependencies to make it easily applicable.
Required parameters:
files: a list of filenames to be plotted.
name: a list of labels for the experiment.
chr: chromosome to be plotted.
output: prefix for the output file.
Optional parameters:
verbose: print version and arguments into a file
histograms: a list of filenames to be plotted as histogram.
histLabels: a list of labels for the histograms.
fillHist: a list whether each histogram will be filled (1) or not (0:default).
histMax : a list of integer for maximum values of histograms.
start: retain after x-th bin (0:default).
end: continues until x-th bin (default: length of the matrix).
resolution: resolution of the bins (default: 100000).
tilePlots: a list of filenames to be plotted as tile plots.
tileLabels: a list of labels for the tile plots.
tileColors: a list of hexadecimal numbers for coloring the tile plots.
tileText: an integer whether text will be displayed above tiles (0:default) or not (1).
arcPlots: a list of filenames to be plotted as arc plots.
arcLabels: a list of labels for the arc plots.
arcColors: a list of hexadecimal numbers for coloring the arc plots.
highlights: an integer for enabling highlights on the plot (0:default), enable(1).
highFile: a file name for a bed file to highlight selected intervals.
peakFiles : a list of filenames to be plotted on the matrix.
window: an integer of distance to calculate insulation score.
tadRange: an integer of window to calculate local minima for TAD calls.
fileHeader: an integer for how many lines should be ignored in the matrix file (1:default).
fileFooter: an integer for how many lines should be skipped at the end of the matrix file (0:default).
smoothNoise: a floating-point number to clean noise in the data.
heatmapColor: an integer for choosing heatmap color codes: Greys(0), Reds(1), YellowToBlue(2), YellowToRed(3-default), Hot(4), BlueToRed(5).
cleanNANs: an integer for replacing NaNs in the matrix with zeros (1:default) or not (0).
plotTriangular: an integer for plotting rotated half matrix (1:default) or not (0).
plotTadDomains: an integer for plotting TADs identified by HiCPlotter (1) or not (0:default).
plotPublishedTadDomins: an integer for plotting TADs from Dixon et, al. 2012 (1:default) or not (0).
plotDomainsAsBars: an integer for plotting TADs as bars (1) instead of triangles (0:default)
highResolution: an integer whether plotting high resolution (1:default) or not (0).
plotInsulation: an integer for plotting insulation scores (0:default) or plot (1).
randomBins: an integer for plotting random resolution data (1:default) or not (0).
wholeGenome: an integer for plotting whole genome interactions (1:default) or not (0).
plotCustomDomains: a list of file names to be plotted beneath the matrix.
publishedTadDomainOrganism : an integer for plotting human (1:default) or mouse (0) TADs from Dixon et, al. 2012.
customDomainsFile: a list of filenames to be plotted as TADs for each experiments.
For visualizing Hi-C or 5C data, HiCPlotter requires a matrix file (by default first line is ignored).
Matrix files are plotted as their log2 values and color legend is put below the plot.
Bin1 Bin2 Bin3 Bin4 Bin5 Bin6
7.85957 4.80329 11.4766 9.57416 4.5288 8.55022
8.61621 4.98956 2.35654 5.69483 11.1187 10.1322
4.06803 4.07801 7.98047 2.59144 6.3851 7.74306
4.52869 2.70624 8.94544 4.29185 8.29491 8.38257
2.91472 3.84658 1.56752 4.48515 7.4955 8.77461
3.08096 2.96487 7.23623 2.33142 3.08529 5.5379
3.12141 3.06905 4.97247 2.39298 5.03621 7.22344
3.4037 2.26455 1.48176 1.41958 3.40252 7.7027
3.8696 1.41425 7.68872 2.21027 5.06846 3.20063
If your file (example is modified from GSM873926) has several header lines, use -fh parameter (this particular case -fh 5).
# resultName mESCs-female-PGK-day2-Replicate1
#
#
#
REV_2|mm9|chrX:98831149-98834145 REV_4|mm9|chrX:98837507-98840771 REV_6|mm9|chrX:98841228-98843248 REV_12|mm9|chrX:98855723-98862021
936.010581657246 743.499513378904 241.956223702097 23.2451328286973
69.8831429744098 513.412096905019 747.143877424081 7.1902317648089
If your file is in bed file format as in GSM1081531, you can covert it to a matrix file with bedToMatrix.py in Utils directory. Each file should have data from one chromosome. To split the file, you can use -> awk '{if($1=="chr6") print}' [file] > [newfile]
chr6 80000_120000 120000_160000 10.278
chr6 80000_120000 160000_200000 3.648
chr6 80000_120000 200000_240000 4.204
*run python bedToMatrix.py [file_name]
For visualizing any type of genomic data, HiCPlotter uses bedGraph format.
chromA chromStartA chromEndA dataValueA color text
chr1 10000 10500 10.0 250,13,27 Polycomb
4th column should be a floating number for histograms.
5th column should be an rgb color for tile or arc plots.
6th column should be a string for tile plots. Columns after 6th will be ignored.
For annotating the interaction matrix, HiCPlotter requires the following format.
chromA chromStartA chromEndA chromB chromStartB chromEndB color (optional)
chr10 100180000 100190000 chr10 100410000 100420000 0,255,255
chr10 101600000 101610000 chr10 101800000 101810000 0,255,255
chr10 102100000 102105000 chr10 102190000 102195000 0,255,255
python HiCPlotter.py -f file1 file2 -n name1 name2 -chr chrX -o output
You can generate all of the following examples by using "sh testRun.sh", all of required files are in the data folder.
Please note: -fh is set to 0 as the input matrix doesn't have a header line.
Hi-C and TADs data taken from: Dixon et, al. Nature 2012
python HiCPlotter.py -f data/HiC/Human/hES-nij.chr21.2 -n hES -chr chr21 -r 40000 -o default1 -fh 0
Start and end locations can be specified as bin numbers with -s and -e parameters.
Color of triangles specify interaction frequency in a given TAD.
TADs identified by Dixon et al. can be plotted with -pptd parameter.
TADs can be plotted as bars instead of triangles with -pdb parameter.
python HiCPlotter.py -f data/HiC/Human/hES-nij.chr21.2 -n hES -chr chr21 -r 40000 -o default2 -ptd 1 -pptd 1 -s 600 -e 900 -fh 0 -w 8 -tr 10 -pi 1
python HiCPlotter.py -f data/HiC/Human/hES-nij.chr21.2 -n hES -chr chr21 -r 40000 -o default2 -ptd 1 -pptd 1 -s 600 -e 900 -fh 0 -w 8 -tr 10 -pi 1 -pdb 1
Hi-C data taken from: Zuin et, al. PNAS 2014
Color code of the heatmaps can be changed with -hmc parameter
python HiCPlotter.py -f data/HiC/Human/GSM1081526_TEV_r1_cis.index.chr6.txt_matrix.txt data/HiC/Human/GSM1081528_HRV_r1_cis.index.chr6.txt_matrix.txt data/HiC/Human/GSM1081530_CTRL_r1_cis.index.chr6.txt_matrix.txt data/HiC/Human/GSM1081533_CTCF_r2_cis.index.chr6.txt_matrix.txt -n WT RAD21-Depleted siControl CTCF-Depleted -chr chr6 -r 40000 -fh 0 -pi 0 -sn 0.35 -o Rad21.CTCF -s 2800 -e 2950 -hmc 5
Multiple histograms for the same matrix should be seperated by comma (true for hist labels and fill histogram parameters).
Data taken from: 4C : Noordermer et, al. Elife 2014, Hi-C and TADs : Dixon et, al. Nature 2012 and CTCF : Stadler et, al. Nature 2011
python HiCPlotter.py -f data/HiC/Mouse/mES.chr2 -n mES -chr chr2 -r 40000 -o HoxD -hist data/HiC/Mouse/GSM1334415_4C_Mouse_EScells_Hoxd4_smoothed_11windows.bedGraph,data/HiC/Mouse/GSM1334440_4C_Mouse_E9.5TB_Hoxd4_smoothed_11windows.bedGraph,data/HiC/Mouse/GSM1334412_4C_Mouse_EScells_Hoxd13_smoothed_11windows.bedGraph,data/HiC/Mouse/GSM1334437_4C_Mouse_E9.5TB_Hoxd13_smoothed_11windows.bedGraph,data/HiC/Mouse/GSM747534_ChIPseq_CTCF_ES_rep1.chr2.bedGraph -hl Hoxd4-ES,Hoxd4-Tail,Hoxd13-ES,Hoxd13-Tail,CTCF-ES -s 1830 -e 1880 -fh 0 -pi 0 -pcd 1 -pcdf data/mES_domains_mm9.bed -fhist 1,1,1,1,0 -hm 2000,2000,2000,2000,50
Data taken from: RAP-seq : Engreitz et al. Science 2014, Hi-C : Dixon et, al. Nature 2012 and H3K27me3 : Mouse ENCODE Project
Rotated matrix can be removed with -ptr 0 parameter
python HiCPlotter.py -f data/HiC/Mouse/mES.chrX -n mES -r 40000 -chr chrX -o RAP -fh 0 -hist data/HiC/Mouse/GSE46918_pSM33-0hr-Xist_vs_Input.W10000_O7500.bedGraph,data/HiC/Mouse/GSE46918_pSM33-1hr-Xist_vs_Input.W10000_O7500.bedGraph,data/HiC/Mouse/GSE46918_pSM33-2hr-Xist_vs_Input.W10000_O7500.bedGraph,data/HiC/Mouse/GSE46918_pSM33-3hr-Xist_vs_Input.W10000_O7500.bedGraph,data/HiC/Mouse/GSE46918_pSM33-6hr-Xist_vs_Input.W10000_O7500.bedGraph,data/HiC/Mouse/wgEncodeLicrHistoneEsb4H3k27me3ME0C57bl6StdSig.chrX.bedGraph -hl Xist_0h,Xist_1h,Xist_2h,Xist_3h,Xist_6h,H3K27me3_0h -pi 0 -ptr 0 -fhist 0,1,1,1,1,0 -hmc 4 -sn 0
Arc plots require a bedGraph file (-a file1), color can be specied as a hexadecimal number (-ac B4B4B4) or for each arc by specified RGB colors in bedGraph file.
Data taken from: SMC ChIA-Pet and Polycomb Domains: Dowen et, al. Cell 2014, Hi-C and TADs : Dixon et, al. Nature 2012 and H3K27me3 : Mouse ENCODE Project
python s.py -f data/HiC/Mouse/mES.chr3 -n mES -chr chr3 -o Bhlhe22 -r 40000 -s 400 -e 500 -a data/HiC/Mouse/mESC_SMC_ChIPPet.bed -al SMC -hist data/HiC/Mouse/GSM747534_chr3.bedGraph,data/HiC/Mouse/wgEncodeLicrHistoneEsb4H3k27me3ME0C57bl6StdSig.chr3.bedGraph -hl CTCF,H3K27me3 -pi 0 -ptr 0 -t data/HiC/Mouse/mm9_Polycomb_domains.bed -tl Polycomb -tc 00CCFF -ac B4B4B4 -fh 0
If bedGraph file for tile plotting contains text in 6th column, features can be plotted above tiles with -tt parameter.
Data taken from: 4C : Lonfat et, al. Science 2014 (Primer locations in Lonfat et, al. are extended to make them more visible in the plot), Hi-C and TADs : Dixon et, al. Nature 2012
python HiCPlotter.py -f data/HiC/Mouse/mES.chr6 -n mES -chr chr6 -r 40000 -o Digit.vs.GT -s 1295 -e 1338 -hist data/HiC/Mouse/GSM1524258_segToFrag_4C_Digits_WT_E12-5_HoxA13_smoothed_11FragsPerWin.bedGraph,data/HiC/Mouse/GSM1524259_segToFrag_4C_GT_WT_E15-5_HoxA13_smoothed_11FragsPerWin.bedGraph -hl Digits,GT -fhist 1,1 -fh 0 -pi 0 -hm 1500,1500 -pcd 1 -pcdf data/mES_domains_mm9.bed -sn 0.4 -t data/HiC/Mouse/primers.bedGraph -tl Enhancers -tt 1
Highlights on the plots can be drawn with -high 1 and passing a bed file name to -hf parameter.
Data taken from: Hi-C and Arrow domains : Rao et, al. Cell 2014, ChIA-Pet Heidari et, al. Genome Research 2014, TADs : Dixon et, al. Nature 2012, ChIP-Seq and Repli-Seq data : Encode Project
python HiCPlotter.py -f data/HiC/Human/GM12878-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/K562-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt -n GM12878 K562 -chr chr10 -r 25000 -pi 0 -fh 0 -o ChIA -a data/HiC/Human/GM12878.Rad21.bed data/HiC/Human/K562.Rad21.bed -al ChIA-PET ChIA-PET -s 3000 -e 3500 -pcd 1 -pcdf data/HiC/Human/GM12878_Arrowhead_domainlist.bed data/HiC/Human/K562_Arrowhead_domainlist.bed -hist data/HiC/Human/wgEncodeUwDnaseGm12878RawRep2.chr10.bedGraph,data/HiC/Human/wgEncodeBroadHistoneGm12878CtcfStdSig.chr10.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqGm12878WaveSignalRep1.bedGraph data/HiC/Human/wgEncodeUwDnaseK562RawRep2.chr10.bedGraph,data/HiC/Human/wgEncodeBroadHistoneK562CtcfStdSig.chr10.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqK562WaveSignalRep1.bedGraph -hl DNAse,CTCF,RepliSeq DNAse,CTCF,RepliSeq -fhist 0,0,1 0,0,1 -pptd 1 -high 1 -hf data/HiC/Human/highlight.bed
Annotations on the matrix can be drawn with -peak parameter.Input file should contain at least six columns.
Data taken from: Hi-C and HiCCUP peaks : Rao et, al. Cell 2014
python HiCPlotter.py -f data/HiC/Human/GM12878-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/KBM7-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/K562-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/HUVEC-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/IMR90-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/HMEC-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/NHEK-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt -n GM12878 KBM7 K562 HUVEC IMR90 HMEC NHEK -r 25000 -pi 0 -fh 0 -o Loops -chr chr10 -peak data/HiC/Human/GSE63525_GM12878_replicate_HiCCUPS_looplist.bed data/HiC/Human/GSE63525_KBM7_HiCCUPS_looplist.bed data/HiC/Human/GSE63525_K562_HiCCUPS_looplist.bed data/HiC/Human/GSE63525_HUVEC_HiCCUPS_looplist.bed data/HiC/Human/GSE63525_IMR90_HiCCUPS_looplist.bed data/HiC/Human/GSE63525_HMEC_HiCCUPS_looplist.bed data/HiC/Human/GSE63525_NHEK_HiCCUPS_looplist.bed -s 3600 -e 3675 -ptr 0
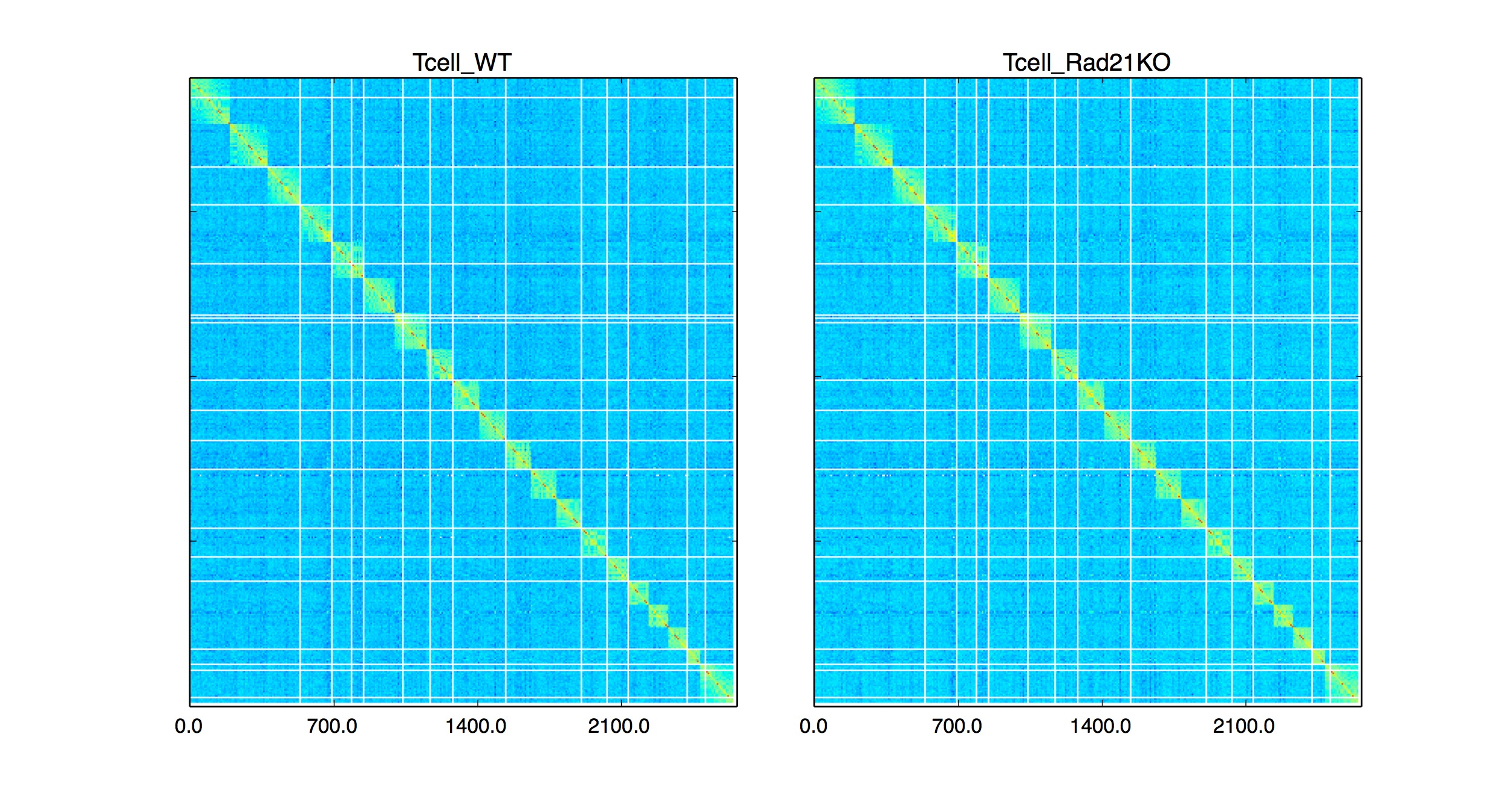
Whole genome plotting can be activated by -wg parameter (Please note: currently only matrixes can be plotted with this option).
Data taken from: Hi-C : Seitan et, al. Genome Research 2014
python HiCPlotter.py -f data/HiC/Human/GSM1184323-HiCMYZ-Tcell-Rad21WT-R1.mm9.NA.L-1400000-wDiag-noSS-iced.2.matrix data/HiC/Human/GSM1184321-HiCMYZ-Tcell-Rad21KO-R1.mm9.NA.L-1400000-wDiag-noSS-iced.2.matrix -n Tcell_WT Tcell_Rad21KO -chr Genome -r 1400000 -o Tcell -pi 0 -ptr 0 -wg 1 -hmc 5 -fh 4
Random binned 5C data plotting can be activated by -rb parameter (Please note: currently only matrixes and triangular plots can be plotted with this option).
Data taken from: 5C data Nora et, al. Nature 2012
python HiCPlotter.py -f data/5C/GSM873926_mESCs-female-PGK12.1-day2-Replicate1.txt data/5C/GSM873932_femaleXO-mESCs-DXTX-replicate-1.matrix.txt data/5C/GSM873924_female-MEFs-replicate-1.matrix.txt -n mESC mESC_XO MEF -fh 8 -chr chrX -o 5C -sn 2 -pi 0 -rb 1 -e 300 -hmc 5
python HiCPlotter.py -f data/HiC/Human/GM12878-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/K562-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/HUVEC-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/NHEK-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/IMR90-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt -n GM12878 K562 HUVEC NHEK IMR90 -chr chr10 -r 25000 -s 3000 -e 3250 -o Figure1 -fh 0
python HiCPlotter.py -f data/HiC/Human/GM12878-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/K562-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/HUVEC-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/NHEK-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/IMR90-chr10_25kb.RAWobserved_KRnormalizedMatrix.txt -n GM12878 K562 HUVEC NHEK IMR90 -chr chr10 -r 25000 -s 3000 -e 3500 -o Figure2 -hist data/HiC/Human/wgEncodeUwDnaseGm12878RawRep2.chr10.bedGraph,data/HiC/Human/wgEncodeBroadHistoneGm12878CtcfStdSig.chr10.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqGm12878WaveSignalRep1.bedGraph data/HiC/Human/wgEncodeUwDnaseK562RawRep2.chr10.bedGraph,data/HiC/Human/wgEncodeBroadHistoneK562CtcfStdSig.chr10.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqK562WaveSignalRep1.bedGraph data/HiC/Human/wgEncodeUwDnaseHuvecRawRep2.chr10.bedGraph,data/HiC/Human/wgEncodeBroadHistoneHuvecCtcfStdSig.chr10.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqHuvecWaveSignalRep1.bedGraph data/HiC/Human/wgEncodeUwDnaseNhekRawRep2.chr10.bedGraph,data/HiC/Human/wgEncodeBroadHistoneNhekCtcfStdSig.chr10.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqNhekWaveSignalRep1.bedGraph data/HiC/Human/wgEncodeOpenChromDnaseImr90BaseOverlapSignal.chr10.bedGraph,data/HiC/Human/wgEncodeSydhTfbsImr90CtcfbIggrabSig.chr10.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqImr90WaveSignalRep1.bedGraph -fh 0 -fhist 0,0,1 0,0,1 0,0,1 0,0,1 0,0,1 -hl DNAse,CTCF,RepliSeq DNAse,CTCF,RepliSeq DNAse,CTCF,RepliSeq DNAse,CTCF,RepliSeq DNAse,CTCF,RepliSeq -hm 400,600,100 400,600,100 400,600,100 400,600,100 400,600,100 -pcd 1 -pcdf data/HiC/Human/GM12878_Arrowhead_domainlist.bed data/HiC/Human/K562_Arrowhead_domainlist.bed data/HiC/Human/HUVEC_Arrowhead_domainlist.bed data/HiC/Human/NHEK_Arrowhead_domainlist.bed data/HiC/Human/IMR90_Arrowhead_domainlist.txt -t data/HiC/Human/GM12878_18_core_K27ac_dense2.bed data/HiC/Human/K562_18_core_K27ac_dense2.bed data/HiC/Human/HUVEC_18_core_K27ac_dense2.bed data/HiC/Human/NHEK_18_core_K27ac_dense2.bed data/HiC/Human/IMR90_18_core_K27ac_dense2.bed -tl ChromHMM ChromHMM ChromHMM ChromHMM ChromHMM -pptd 1 -high 1 -hf data/HiC/Human/fig2.bed
python HiCPlotter.py -f data/HiC/Human/GM12878-chr15_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/K562-chr15_25kb.RAWobserved_KRnormalizedMatrix.txt -n GM12878 K562 -chr chr15 -r 25000 -s 1800 -e 2250 -o Figure3 -hist data/HiC/Human/wgEncodeUwDnaseGm12878RawRep1.chr15.bedGraph,data/HiC/Human/wgEncodeBroadHistoneGm12878CtcfStdSig.chr15.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqGm12878WaveSignalRep1.bedGraph data/HiC/Human/wgEncodeUwDnaseK562RawRep1.chr15.bedGraph,data/HiC/Human/wgEncodeBroadHistoneK562CtcfStdSig.chr15.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqK562WaveSignalRep1.bedGraph -fh 0 -fhist 0,0,1 0,0,1 -hl DNase,CTCF,RepliSeq DNase,CTCF,RepliSeq -hm 400,400,100 400,400,100 -t data/HiC/Human/GM12878_Enhancer.bed,data/HiC/Human/GM12878_Txn.bed,data/HiC/Human/GM12878_Het.bed data/HiC/Human/K562_Enhancer.bed,data/HiC/Human/K562_Txn.bed,data/HiC/Human/K562_Het.bed -tl Enhancer,Transcribed,Heterochromatin Enhancer,Transcribed,Heterochromatin -a data/HiC/Human/GM12878.Rad21.bed data/HiC/Human/K562.Rad21.bed -al RAD21 RAD21 -ptr 0 -high 1 -hf data/HiC/Human/fig3.bed
python HiCPlotter.py -f data/HiC/Human/GM-chr19_25kb.RAWobserved_KRnormalizedMatrix.txt data/HiC/Human/K562-chr19_25kb.RAWobserved_KRnormalizedMatrix.txt -n GM12878 K562 -r 25000 -chr chr19 -hist data/HiC/Human/GM12878.DNAse.chr19.2.bedGraph,data/HiC/Human/GM12878.RnaSeq.chr19.2.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqGm12878WaveSignalRep1.bedGraph data/HiC/Human/K562.DNAse.chr19.2.bedGraph,data/HiC/Human/K562.RnaSeq.chr19.2.bedGraph,data/HiC/Human/wgEncodeUwRepliSeqK562WaveSignalRep1.bedGraph -hl DNAse,RNASeq,RepliSeq DNAse,RNASeq,RepliSeq -t data/HiC/Human/GM12878_TSS+Trx.2.bed data/HiC/Human/K562_TSS+Trx.2.bed -tl ChromHMM ChromHMM -high 1 -hf data/HiC/Human/region.bed -o Figure4 -s 1100 -e 1302 -hm 300,300,100 300,300,100 -fh 0 -fhist 0,0,1 0,0,1 -ptr 0
*If your data contains several columns before data matrix, from command line you could use:
cut -f N- matrix > new_matrix (where N is the ith column data values start)
*If any of the imported packages are missing in your python system, try to comment out those lines. For example:
Original : from scipy.signal import argrelextrema (line 20)
Try this : #from scipy.signal import argrelextrema (line 20). Use HiCPlotter with the -pi 0 and -ptd 0
*If you received the following error: "IOError: encoder jpeg not available", please change extensions of '.jpeg' to '.png' after line 880.
*If you like to run HiCPlotter in verbose mode, please use -v parameter which will create a log file with which parameters the program ran.
*If you need to convert bigWig files to bedGraph files, you can use kentUtils/bigWigToBedGraph executable.
If you encounter any problems, please contact with - Kadir Akdemir (kcakedemir at mdanderson dot org).
The MIT License (MIT)
Copyright (c) 2015, Kadir C. Akdemir and Lynda Chin
Permission is hereby granted, free of charge, to any person obtaining a copy of this software and associated documentation files (the "Software"), to deal in the Software without restriction, including without limitation the rights to use, copy, modify, merge, publish, distribute, sublicense, and/or sell copies of the Software, and to permit persons to whom the Software is furnished to do so, subject to the following conditions:
The above copyright notice and this permission notice shall be included in all copies or substantial portions of the Software.
THE SOFTWARE IS PROVIDED "AS IS", WITHOUT WARRANTY OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO THE WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE AND NONINFRINGEMENT. IN NO EVENT SHALL THE AUTHORS OR COPYRIGHT HOLDERS BE LIABLE FOR ANY CLAIM, DAMAGES OR OTHER LIABILITY, WHETHER IN AN ACTION OF CONTRACT, TORT OR OTHERWISE, ARISING FROM, OUT OF OR IN CONNECTION WITH THE SOFTWARE OR THE USE OR OTHER DEALINGS IN THE SOFTWARE.
Thanks to Lynda Chin for her leadership, management and support.
Thanks to Zeynep Coban-Akdemir, Ian Watson, Denise Spring, Jason Ernst, Tony Gutschner, Kunal Rai and Samir Amin for their insightful comments.
We are grateful to many researchers cited above for providing their data in publicly available and easy-to-use format.
.40K.jpeg)
.40K.jpeg)
.40K.jpeg)
.40K.jpeg)
.40K.jpeg)
.40K.jpeg)
.40K.jpeg)
.40K.jpeg)
.25K.jpeg)
.25K.jpeg)
.RandomBins.jpeg)
.25K.jpeg)
.25K.jpeg)
.25K.jpeg)
.25K.jpeg)