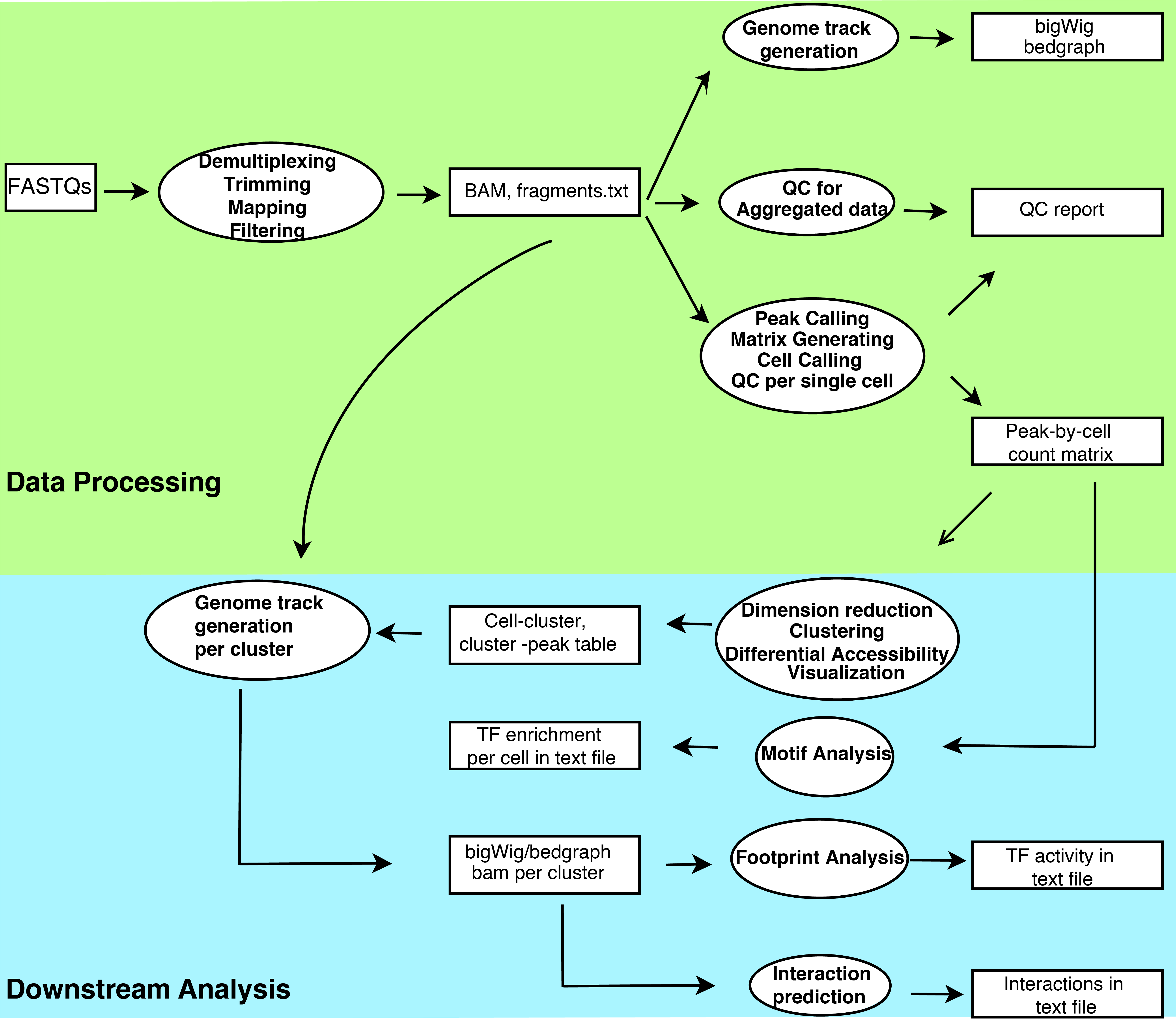
A comprehensive workbench for single cell ATAC-seq data processing, analysis and visualization
scATAC-pro consists of two units, the data processing unit and the downstream analysis unit. The data processing unit takes raw fastq files as input and outputs peak-by-cell count matrix, QC report and genome track files. It consists of the following modules: demultiplexing, adaptor trimming, read mapping, peak calling, cell calling, genome track file generation and quality control assessment. The downstream analysis unit consists of the following modules: dimension reduction, cell clustering, differential accessibility analysis, gene ontology analysis, TF motif enrichment analysis, TF footprinting analysis, linking regulatory DNA sequences with gene promoters, and integration of multiple datasets. We provide flexible options for most of analysis modules.
- Note: It is not necessary to install scATAC-pro from scratch. You can use the docker or singularity version if you prefer (see Run scATAC-pro through docker or singularity )
- Run the following command in your terminal, scATAC-pro will be installed in YOUR_INSTALL_PATH/scATAC-pro_1.1.1
$ git clone https://github.com/wbaopaul/scATAC-pro.git
$ cd scATAC-pro
$ make configure prefix=YOUR_INSTALL_PATH
$ make install
- Current version: 1.1.1
- March, 2020
- get_mtx requires two input files: a fragments.txt file and a peak file, separated by comma
- annotate peak as overlapped with a gene Tss if the corresponding distance <= 1000bp; mark peak with a gene if their distance <= 100kb
- Feb, 2020
- integrate module enables 3 options: seurat, harmony and pool
- new module visualize, allowing interactively explore and analyze the data
- footprint module supports one-vs-rest comparison and provides result in heatmap
- module runDA changed to use group name as the input (e.g. "0:1,2" or "one,rest")
- installed rgt-hint (for footprinting analysis) using miniconda3
- added module process_with_bam, allowing processing from aggregated bam file
- enabled data integration from peaks files, assuming all data sets are processed using scATAC-pro. Output matrix with the same merged peaks/features and the previously called cells, along with an integrated seurat object
- added new parameters in the configuration file: Top_Variable_Features, REDUCTION, nREDUCTION
- enabled all clustering methods mentioned in the manuscript, along with kmeans clustering on principal components
- file path changed to like downstreame_analysis/PEAK_CALLER/CELL_CALLER/..., indicating peak caller
- Jan, 2020
- added a new module mergePeaks to merge different peak files called from different data sets
- added a new module reConstMtx to reconstruct peak-by-cell matrix given a peak file, a fragment file and a barcodes.txt file
- Dec, 2019
- corrected an error due to using older version of chromVAR
- corrected a bug for demultiplexing multiple index files
- added a module convert10xbam to convert 10x position sorted bam file to scATAC-pro file format
- updated module get_bam4Cells, with the inputs as a bam file and a txt file of barcodes, separated by comma
- R (>=3.6.1)
- Python (>=3.6.0)
The following packages will be automatically installed if NOT detected by the installation script.
- BWA (>=0.7.17), bowtie, bowtie2
- MACS2 (>=2.2.5)
- samtools (>=1.9)
- bedtools (>=2.27.1)
- deepTools (>=3.2.1)
- trim_galore (>=0.6.3), Trimmomatic (>=0.6.3)
- Regulratory Genomics Toolbox (RGT, for footprinting analysis, will ask whether you want to install it since the installation is done through conda, which takes a while and you may not want to conduct footprinting analysis)
- g++ compiler, bzip2, ncurses-devel
- R packaages: devtools, flexdashboard, png, data.table, Matirx, Rcpp, ggplot2, flexmix, optparse, magrittr, readr, Seurat, bedr, gridExtra, ggrepel, kableExtra, viridis, xlsx, RColorBrewer,pheatmap,motifmatchr, chromVAR, chromVARmotifs, SummarizedExperiment, BiocParallel, DESeq2, clusterProfiler, BSgenome.Hsapiens.UCSC.hg38, BSgenome.Mmusculus.UCSC.mm10, VisCello.atac
-
IMPORTANT: The parameters and options should be specified in a configurartion file in plain text format. Copy and edit the configure_user.txt file in this repository and then in your terminal run the following commands:
-
NOTE: some mapping index and genome annotation files can be downloaded rgtdata
$ scATAC-pro -s process
-i pe1_fastq,pe2_fastq,index_fastq
-c configure_user.txt
$ scATAC-pro -s downstream
-i output/filtered_matrix/PEAK_CALLER/CELL_CALLER/matrix.mtx
-c configure_user.txt
## PEAK_CALLER and CELL_CALLER is specified in your configure_user.txt file
- For data processing, if fastq files have been demultiplexed as the required format with the barcode recorded in the name of each read as @barcode:ORIGIN_READ_NAME , you can skip the demultiplexing step by running the following command:
$ scATAC-pro -s process_no_dex
-i pe1_fastq,pe2_fastq
-c configure_user.txt
- The output will be saved under ./output as default
- --verbose (or -b) will print the running message on screen, otherwise the message will only be saved under output/logs/MODULE.txt
-
IMPORTANT: you can run scATAC-pro sequentially. The input of a later analysis module is the output of the previous analysis modules. The following tutorial uses fastq files downloaded from PBMC10k 10X Genomics
-
Combine data from different sequencing lanes
$ cat atac_pbmc_10k_v1_S1_L001_R1_001.fastq.gz atac_pbmc_10k_v1_S1_L002_R1_001.fastq.gz > pe1_fastq.gz
$ cat atac_pbmc_10k_v1_S1_L001_R3_001.fastq.gz atac_pbmc_10k_v1_S1_L002_R3_001.fastq.gz > pe2_fastq.gz
$ cat atac_pbmc_10k_v1_S1_L001_R2_001.fastq.gz atac_pbmc_10k_v1_S1_L002_R2_001.fastq.gz > index_fastq.gz
- Run scATAC-pro sequentially
$ scATAC-pro -s demplx_fastq
-i pe1_fastq.gz,pe2_fastq.gz,index_fastq.gz
-c configure_user.txt
$ scATAC-pro -s trimming
-i output/demplxed_fastq/demplxed_pe1_fastq.gz,
output/demplxed_fastq/demplxed_pe2_fastq.gz
-c configure_user.txt
$ scATAC-pro -s mapping
-i output/trimmed_fastq/trimmed_pe1_fastq.gz,
output/trimmed_fastq/trimmed_pe2_fastq.gz
-c configure_user.txt
$ scATAC-pro -s call_peak
-i output/mapping_result/pbmc10k.positionsort.MAPQ30.bam
-c configure_user.txt
$ scATAC-pro -s aggr_signal
-i output/mapping_result/pbmc10k.positionsort.MAPQ30.bam
-c configure_user.txt
$ scATAC-pro -s get_mtx
-i output/summary/pbmc10k.fragments.txt,output/peaks/MACS2/pbmc10k_features_BlacklistRemoved.bed
-c configure_user.txt
$ scATAC-pro -s qc_per_barcode
-i output/summary/pbmc10k.fragments.txt,output/peaks/MACS2/pbmc10k_features_BlacklistRemoved.bed
-c configure_user.txt
$ scATAC-pro -s call_cell
-i output/raw_matrix/PEAK_CALLER/matrix.mtx
-c configure_user.txt
$ scATAC-pro -s get_bam4Cells
-i output/mapping_result/pbmc10k.positionsort.bam,
output/filtered_matrix/PEAK_CALLER/CELL_CALLER/barcodes.txt
-c configure_user.txt
## after running the above module, you can run module report (list below)
## to generate first page of the summary report
$ scATAC-pro -s clustering
-i output/filtered_matrix/PEAK_CALLER/CELL_CALLER/matrix.mtx
-c configure_user.txt
$ scATAC-pro -s motif_analysis
-i output/filtered_matrix/PEAK_CALLER/CELL_CALLER/matrix.mtx
-c configure_user.txt
$ scATAC-pro -s split_bam
-i output/downstream_analysis/PEAK_CALLER/CELL_CALLER/cell_cluster_table.txt
-c configure_user.txt
$ scATAC-pro -s footprint ## supporting comparison two clusters, and one-vs-rest
-i 0,1 ## or '0,rest' (means cluster1 vs rest) or 'one,rest' (all one-vs-rest)
-c configure_user.txt
$ scATAC-pro -s runDA
-i 0:1:3,2 ## group1 consist of cluster 0,1,and 3; group2 cluster2
-c configure_user.txt
$ scATAC-pro -s runGO
-i output/filtered_matrix/PEAK_CALLER/CELL_CALLER/differential_peak_cluster_table.txt
-c configure_user.txt
$ scATAC-pro -s report
-i output/summary
-c configure_user.txt
## merge peaks that are within 200bp distance of each other
$ scATAC-pro -s mergePeaks
-i peak_file1,peak_file2,(peak_file3...),200
-c configure_user.txt
## perform integrated analysis, assuming all data sets are processed by scATAC-pro
## which means each fragments.txt and barcodes.txt files can be found correspondingly
$ scATAC-pro -s integrate
-i peak_file1,peak_file2,(peak_file3...) ##
-c configure_user.txt
## if you have the reconstructed matrix for data set (meaning using the merged peaks)
## you can run the *integrate_seu* whtich is second part of the module *integrate*
$ scATAC-pro -s integrate_seu
-i mtx_file1,mtx_file2,(mtx_file3...)
-c configure_user.txt
- After clustering, user can interactively visualize and analyze the data with module visualize
scATAC-pro -s visualize -i output/downstream_analysis/PEAK_CALLER/CELL_CALLER/VisCello_obj -c configure_user.txt
- Note that the visualization can also be done through R/Rstudio:
devtools::install_github("qinzhu/VisCello", ref="VisCello-atac") ## install the package
library(VisCello.atac)
cello('output/downstream_analysis/PEAK_CALLER/CELL_CALLER/VisCello_obj') ## launch VisCello in your web browser with prepared data
- More details about the visualization module can be found at VisCello
$ scATAC-pro --help
usage : scATAC-pro -s STEP -i INPUT -c CONFIG [-o] [-h] [-v]
Use option -h|--help for more information
scATAC-pro 1.1.1
---------------
OPTIONS
[-s|--step ANALYSIS_STEP] : run an analysis module (or some combination of several modules) of the scATAC-pro workflow, supported modules include:
demplx_fastq: perform demultiplexing
input: fastq files for both reads and index, separated by comma like:
PE1_fastq,PE2_fastq,index1_fastq,inde2_fastq,index3_fastq...
output: Demultiplexed fastq1 and fastq2 files with index information embedded
in the read name as: @index3_index2_index1:original_read_name, saved in
output/demplxed_fastq/
trimming: trim read adapter
input: demultiplexed fastq1 and fastq2 files
output: trimmed demultiplexed fastq1 and fastq2 files, saved in output/trimmed_fastq/
mapping: perform reads alignment
input: fastq files, separated by comma for each paired end
output: position sorted bam file saved in output/mapping_result, mapping qc stat and
fragment.txt files saved in output/summary
call_peak: call peaks using aggregated data
input: BAM file, outputted from the mapping module
output: peaks in plain text format, saved as output/peaks/PEAK_CALLER/
OUTPUT_PREFIX_features_Blacklist_Removed.bed
get_mtx: build raw peak-by-cell matrix
input: fragment.txt file, outputted from the mapping module, and features/peak file,
outputted from the call_peak module, separated by a comma
output: sparse peak-by-cell count matrix in Matrix Market format, barcodes and feature files
in plain text format, saved in output/raw_matrix/PEAK_CALLER/
aggr_signal: generate aggregated signal, which can be uploaded to and viewed
in genome browser
input: BAM file, outputted from the mapping module
output: Aggregated data in .bw and .bedgraph file, saved in output/signal/
qc_per_barcode: generate quality control metrics for each barcode
input: fragment.txt file (outputted from module mapping) and peak/feature file,
(outputted from module call_peak), separated by comma
output: qc_per_barcode.txt file, saved in output/summary/
call_cell: perform cell calling
input: raw peak-by-barcode matrix file, outputted from the get_mtx module
output: filtered peak-by-cell matrix in Market Matrix format, barcodes and features,
saved in output/filtered_matrix/PEAK_CALLER/CELL_CALLER/
get_bam4Cells: extract bam file for cell barcodes and calculate mapping stats correspondingly
input: A bam file for aggregated data outputted from mapping module and a barcodes.txt file
outputted from module call_cell, separated by comma
output: A bam file saved in output/mapping_results and mapping stats (optional) saved
in output/summary for cell barcodes
process: processing data - including demplx_fastq, mapping, call_peak, get_mtx,
aggr_signal, qc_per_barcode, call_cell and get_bam4Cells
input: fastq files for both reads and index, separated by comma like:
fastq1,fastq2,index_fastq1,index_fastq2, index_fastq3...;
output: peak-by-cell matrix and all intermediate results
process_no_dex: processing data without demultiplexing
input: demultiplexed fastq files for both reads and index, separated by comma like:
fastq1,fastq2;
output: peak-by-cell matrix and all intermediate results
process_with_bam: processing from bam file
input: bam file for aggregated data, outputted from the mapping module
output: filtered peak-by-cell matrix and all intermediate results
clustering: cell clustering
input: filtered peak-by-cell matrix file, outputted from the call_cell module
output: seurat objects with clustering label in the metadata (.rds file) and
barcodes with cluster labels (cell_cluster_table.txt file), and umap plot colorred
clustering label, saved in output/downstream_analysiss/PEAK_CALLER/CELL_CALLER/
motif_analysis: perform TF motif analysis
input: filtered peak-by-cell matrix file, outputted from the call_cell module
output: TF-by-cell enrichment matrix in chromVAR object, a table and heatmap indicating
TF enrichment for each cell cluster, saved in output/downstream_analysiss/
PEAK_CALLER/CELL_CALLER/
runDA: preform differential accessibility analysis
input: either two groups named as '0:1,2' in which group1 consists of cluster 0 and 1,
and group2 consists of cluster2 or specified as '0,rest', or 'one,rest'
output: differential accessibility peaks in txt format saved in the same in
output/downstream_analysiss/PEAK_CALLER/CELL_CALLER/
runGO: preform GO term enrichment analysis
input: differential accessible features file, outputted from runDA module (.txt file)
output: enriched GO terms in .xlsx format saved in the same directory as the input file
runCicero: run cicero for calculating gene activity score and predicting cis chromatin interactions
input: seurat_obj.rds file outputted from the clustering module
output: cicero gene activity in .rds format and predicted interactions in .txt format, saved
in output/downstream_analysiss/PEAK_CALLER/CELL_CALLER/
split_bam: split bam file into different clusters
input: barcodes with cluster label (cell_cluster_table.txt file, outputted from
clustering module
output: .bam file (saved in output/downstream/PEAK_CALLER/CELL_CALLER/data_by_cluster),
.bw, .bedgraph (saved in output/signal/) file for each cluster
footprint: perform TF footprinting analysis, supports comparison between two clusters and one cluster vs
the rest of cell clusters (one-vs-rest)
input: 0,1 ## or '0,rest' (means cluster1 vs rest) or 'one,rest' (all one-vs-rest)
output: footprinting summary statistics in tables and heatmap,
saved in output/downstream/PEAK_CALLER/CELL_CALLER/
downstream: perform all downstream analyses, including clustering, motif_analysis,
split_bam (optional) and footprinting analysis (optional)
input: filtered peak-by-cell matrix file, outputted from call_cell module
output: all outputs from each module
report: generate summary report in html file
input: directory to QC files, output/summary as default
output: summary report in html format, saved in output/summary and .eps figures for each panel
saved in output/summary/Figures
convert10xbam: convert bam file in 10x genomics format to bam file in scATAC-pro format
input: bam file (position sorted) in 10x format
output: position sorted bam file in scATAC-pro format saved in output/mapping_result,
mapping qc stat and fragment.txt files saved in output/summary/
mergePeaks: merge peaks (called from different data sets) if the distance is
less than a given #basepairs (200 if not specified)
input: peak files and a distance parameter separated by comma:
peakFile1,peakFile2,peakFile3,200
output: merged peaks saved in file output/peaks/merged.bed
reconstMtx: reconstruct peak-by-cell matrix given peak file, fragments.txt file, and barcodes.txt file
input: different files separated by comma:
peakFilePath,fragmentFilePath,barcodesPath
output: a sub-folder reConst_matrix for the reconstructed peak-by-cell matrix, saved in
the same path as the input barcodes.txt file
integrate: perform integration of two ore more data sets
input: peak/feature files, separated by comma: peak_file1,peak_file2
output: merged peaks, reconstructed matrix, integrated seurat obj and umap plot, saved in
output/integrated/
integrate_seu: perform integration of two ore more data sets given the reconstructed peak-by-cell matrix
input: mtx1,mtx2, separated by comma like, mtx_file1,mtx_file2
output: integrated seurat obj and umap plot, saved in output/integrated/
visualize: interactively visualize the data through VisCello
input: VisCello_obj directory, outputted from the clustering module
output: launch VisCello through web browser for interactively visualization"
-i|--input INPUT : input data, different types of input data are required for different analysis
-c|--conf CONFIG : configuration file for parameters (if exist) for each analysis module
[-o|--output_dir : folder to save results, default output/ under the current directory; sub-folder will be created automatically for each analysis
[-h|--help]: print help infromation on screen
[-v|--version]: display current version numbe of scATAC-pro on screen
[-b|--verbose]: print running message on screen
In case you have problem in installing dependencies, you can run scATAC-pro without installing dependencies in one of the following ways:
-
Run the pre-built dockerized version, pull the docker image here
-
Run it through singularity (which is more friendly with high performance cluster or HPC, and linux server) by running the following command:
$ singularity pull -F docker://wbaopaul/scatac-pro
## will generate scatac-pro_latest.sif in current directory
$ singularity exec -H YOUR_WORK_DIR --cleanenv scatac-pro_latest.sif scATAC-pro -s XXX -i XXX -c XXX
- To use it on HPC cluster:
# write a script mapping.sh for mapping as an example:
#!/bin/bash
module load singularity
singularity pull -F docker://wbaopaul/scatac-pro ## you just need run line this once
## will generate scatac-pro_latest.sif in the current directory
singularity exec --cleanenv -H /mnt/isilon/tan_lab/yuw1/run_scATAC-pro/PBMC10k scatac-pro_latest.sif \
scATAC-pro -s mapping -i fastq_file1,fastq_file2 -c configure_user.txt
# and then qsub mapping.sh
-
NOTE: YOUR_WORK_DIR is your working directory, where the outputs will be saved and all data under YOUR_WORK_DIR will be available to scATAC-pro
-
NOTE: all inputs including data paths specified in configure_user.txt should be available under YOUR_WORK_DIR
-
NOTE: if running the footprint module, remember to download the reference data rgtdata folder and put it under YOUR_WROK_DIR
- How to proceed using 10x cellranger-atac output?
- How to merge different peaks called from different data sets?
- How to reconstruct peak-by-cell matrix after updating peak file?
- How to access downstream analysis results in R?
Yu W, Uzun Y, Zhu Q, Chen C, Tan K. scATAC-pro: a comprehensive workbench for single-cell chromatin accessibility sequencing data. Genome Biology; 2020