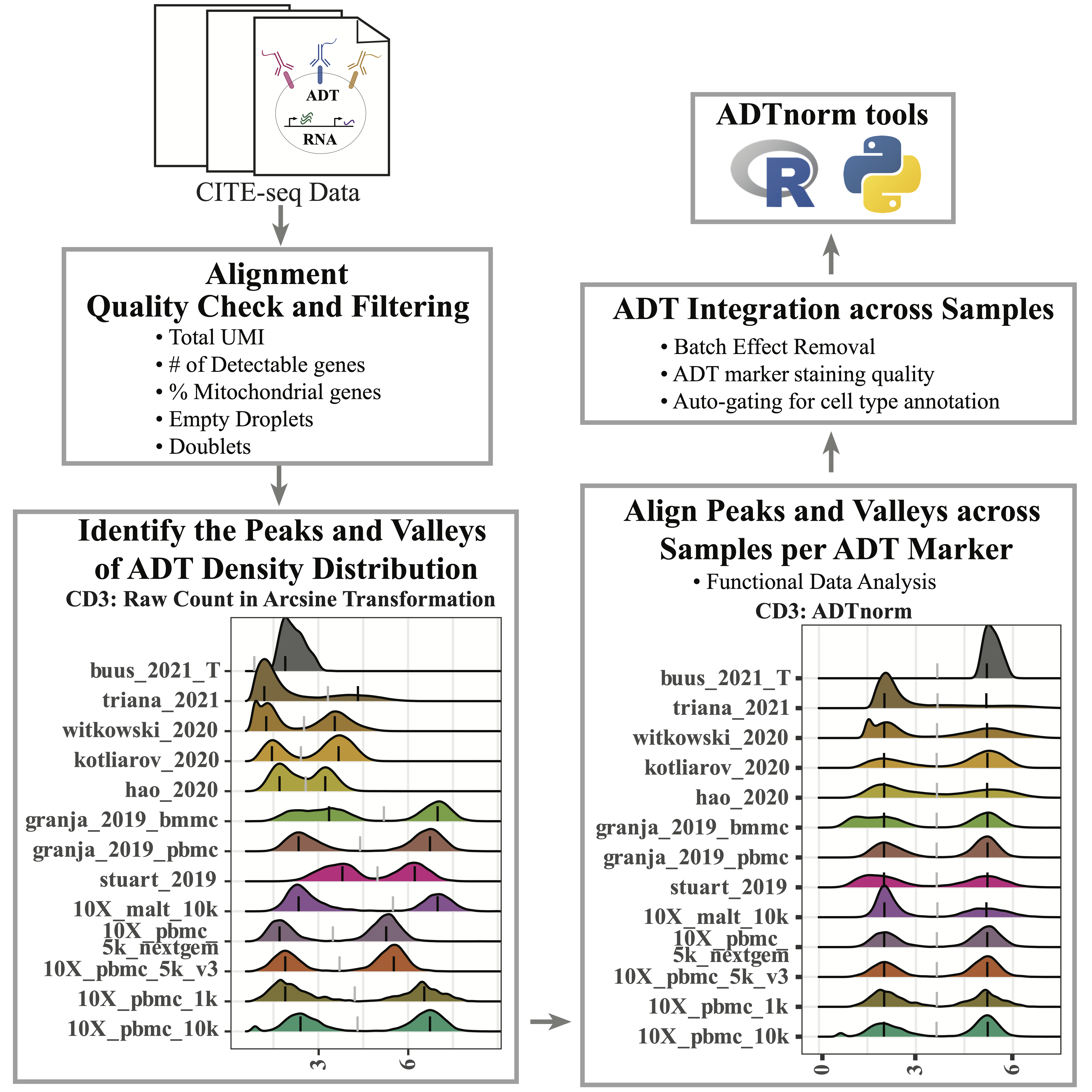
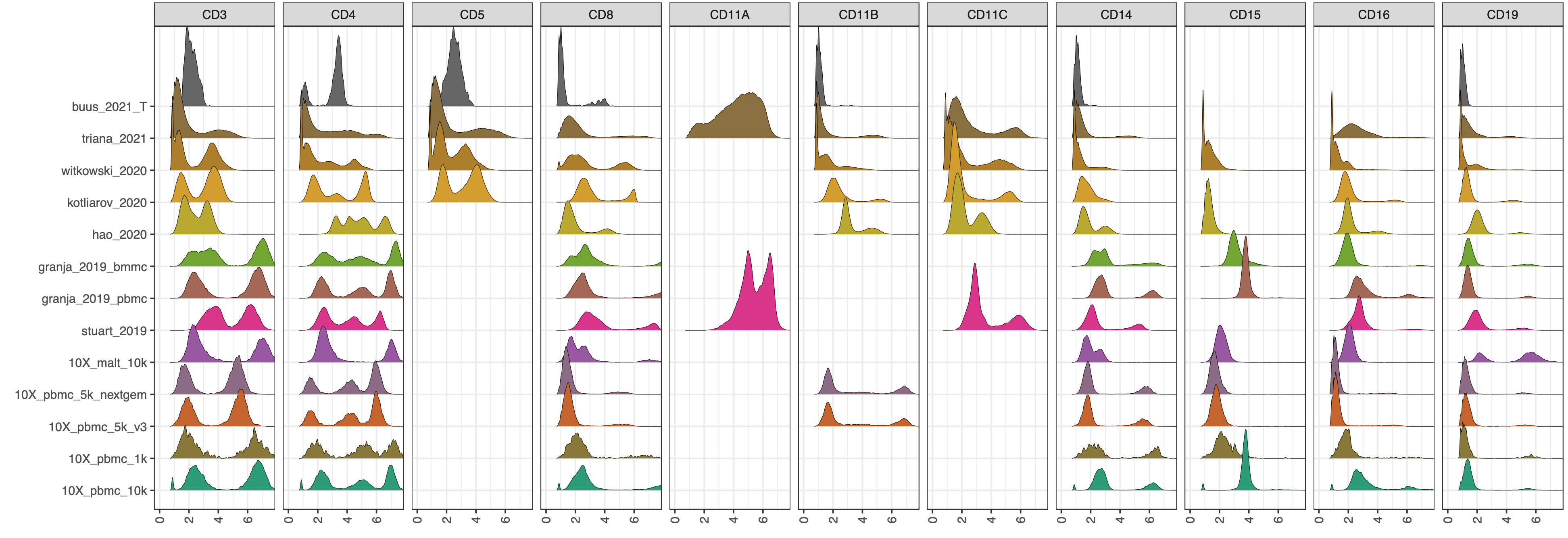
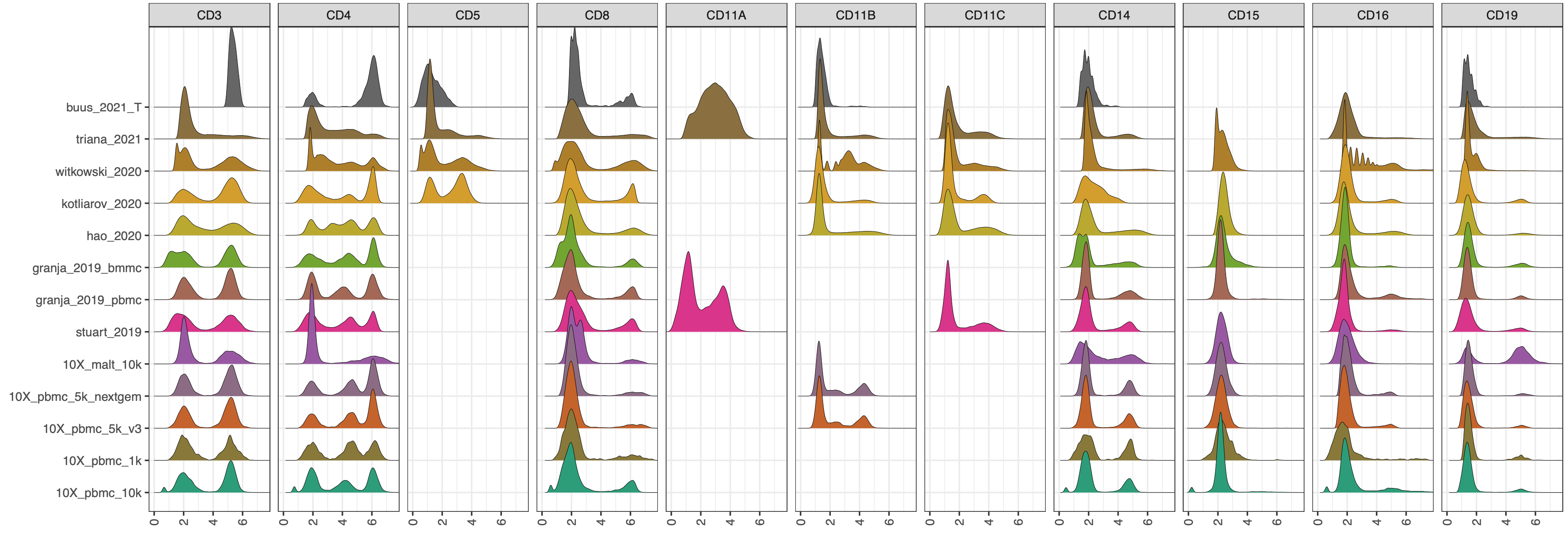
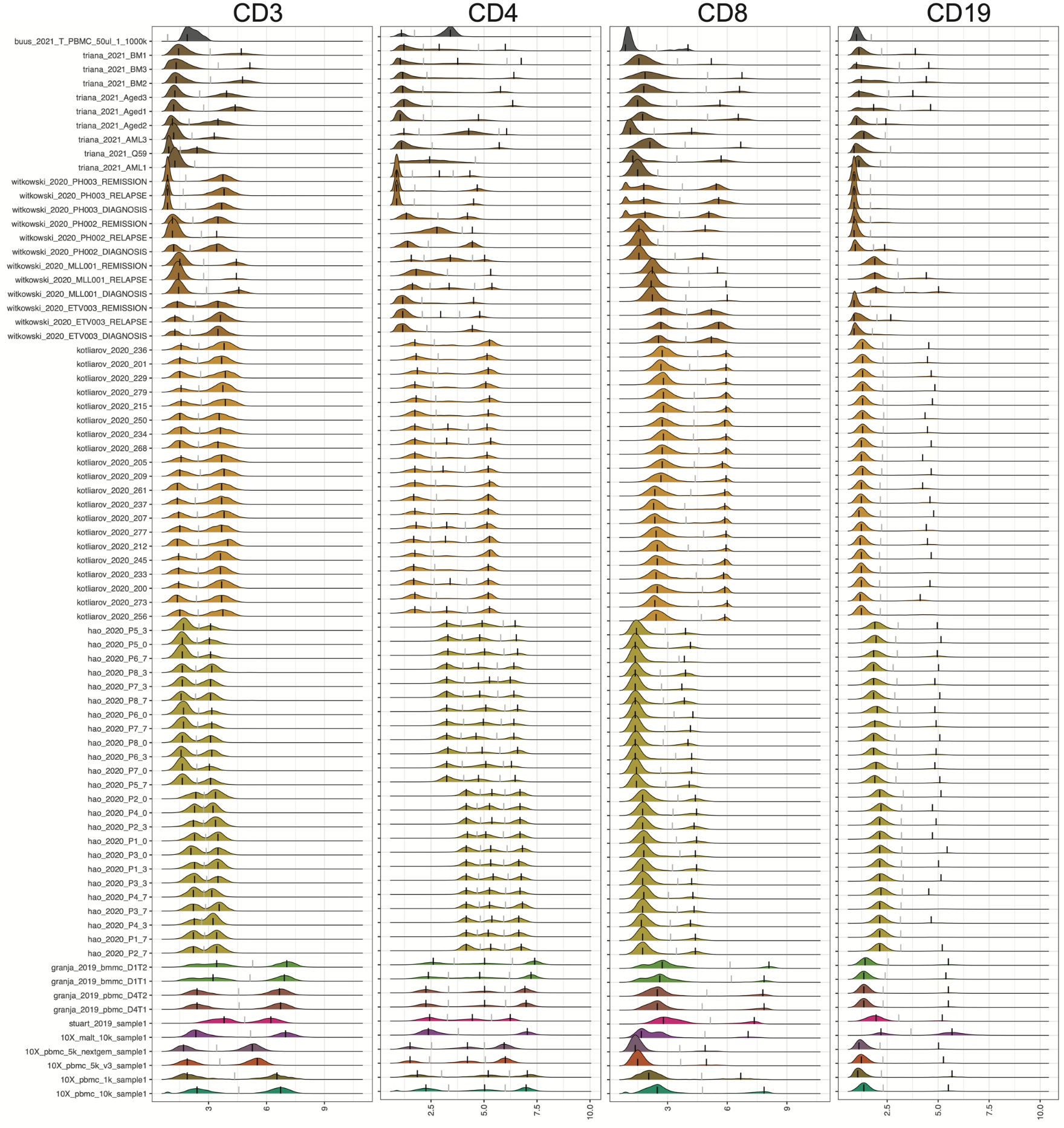
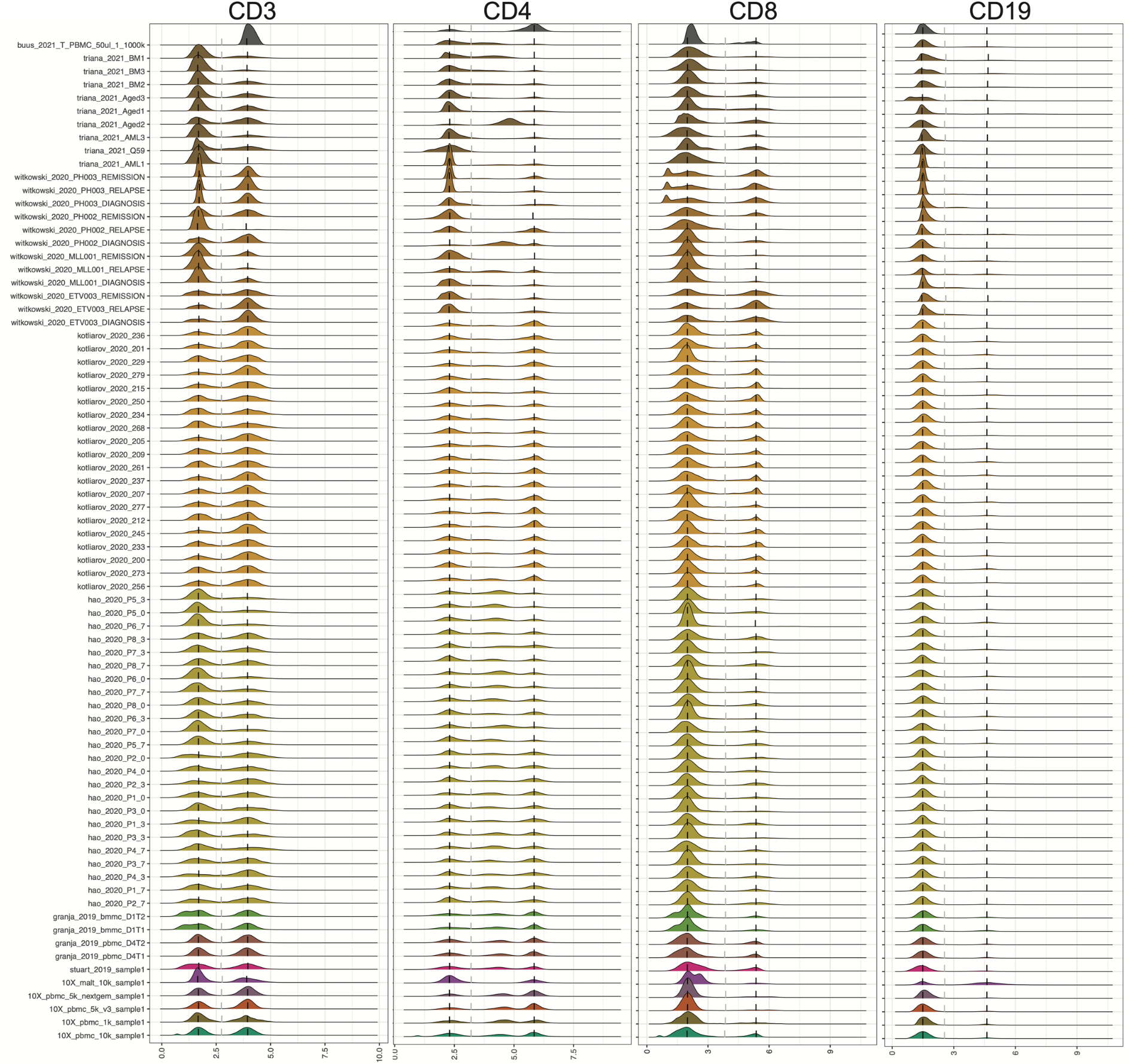
CITE-seq enables paired measurement of surface protein and mRNA expression in single cells using antibodies conjugated to oligonucleotide tags. Due to the high copy number of surface protein molecules, sequencing antibody-derived tags (ADTs) allows for robust protein detection, improving cell-type identification. However, variability in antibody staining leads to batch effects in the ADT expression, obscuring biological variation, reducing interpretability, and obstructing cross-study analyses. Here, we present ADTnorm, a normalization and integration method designed explicitly for ADT abundance. Benchmarking against 14 existing scaling and normalization methods, we show that ADTnorm accurately aligns populations with negative- and positive-expression of surface protein markers across 13 public datasets, effectively removing technical variation across batches and improving cell-type separation. ADTnorm enables efficient integration of public CITE-seq datasets, each with unique experimental designs, paving the way for atlas-level analyses. Beyond normalization, ADTnorm aids in automated threshold-gating as well as assessment of antibody staining quality for titration optimization and antibody panel selection.
This repository is the ADTnorm R package. We also provide a Python wrapper by Daniel P. Caron.
# install.packages("remotes")
remotes::install_github("yezhengSTAT/ADTnorm", build_vignettes = FALSE)There are many dependencies in ADTnorm, so it takes a long time to install them all. Instead, you can use the Docker image of ADTnorm.
docker pull ghcr.io/yezhengstat/adtnorm:latest
docker run \
-it \
--user rstudio \
--volume <yourDataDirectory>:/home/rstudio/data \
yezhengstat/adtnorm:latest \
RReplace <yourDataDirectory> with the local directory path (absolute path) where you have the input data and would like to store the output files. For more information on using docker containers, please read this documentation by Bioconductor.
The 13 public datasets used in the manuscript are also included in the R package as a demo data set. They can be loaded by
data(cell_x_adt)
data(cell_x_feature)
cell_x_adtcontains raw counts for ADT markers in each cell. It is a data frame with 422682 cells (rows) and 9 ADT markers (columns): CD3, CD4, CD8, CD14, CD19, CD25, CD45RA, CD56, CD127.
CD3 CD4 CD8 CD14 CD19 CD25 CD45RA CD56 CD127
1 18 138 13 491 3 9 110 17 7
2 30 119 19 472 3 5 125 248 8
3 18 207 10 1289 8 15 5268 26 12
4 18 11 17 20 5 15 4743 491 16
5 5 14 14 19 4 16 4108 458 17
6 21 1014 29 2428 7 52 227 29 15
-
cell_x_featureis a data frame with 422682 cells (rows) and 7 feature variables (columns):-
sample: Sample name used in original data of each study.
-
batch: Batch information provided from each study.
-
sample_status: Sample status, i.e., Healthy, MALTtumor, HIV Vaccine, Lupus, B-ALL, AML.
-
study_name: Name of the data set/study.
-
ADTseqDepth: Total UMI per cell.
-
cell_type_l1: Broad level of cell type annotation using manual gating.
-
cell_type_l2: Fine level of cell type annotation using manual gating.
-
sample batch sample_status study_name
1 10X_pbmc_10k_sample1 10X_pbmc_10k_batch1 healthy 10X_pbmc_10k
2 10X_pbmc_10k_sample1 10X_pbmc_10k_batch1 healthy 10X_pbmc_10k
3 10X_pbmc_10k_sample1 10X_pbmc_10k_batch1 healthy 10X_pbmc_10k
4 10X_pbmc_10k_sample1 10X_pbmc_10k_batch1 healthy 10X_pbmc_10k
5 10X_pbmc_10k_sample1 10X_pbmc_10k_batch1 healthy 10X_pbmc_10k
6 10X_pbmc_10k_sample1 10X_pbmc_10k_batch1 healthy 10X_pbmc_10k
ADTseqDepth cell_type_l1 cell_type_l2
1 981 monocytes classical monocyte
2 1475 monocytes classical monocyte
3 7149 monocytes classical monocyte
4 6831 NK CD16+ NK
5 6839 NK CD16+ NK
6 4720 monocytes classical monocyte
For more detailed and typical parameter tuning examples, please visit tutorial website. We will illustrate using the demo data.
library(ADTnorm)
save_outpath <- "/path/to/output/location"
run_name <- "ADTnorm_demoRun"
data(cell_x_adt)
data(cell_x_feature)
cell_x_feature$sample = factor(cell_x_feature$study_name) ## consider each study as one sample
cell_x_feature$batch = factor(cell_x_feature$study_name) ## consider each study as a batch
cell_x_adt_norm <- ADTnorm(
cell_x_adt = cell_x_adt,
cell_x_feature = cell_x_feature,
save_outpath = save_outpath,
study_name = run_name,
marker_to_process = c("CD3", "CD4", "CD8", "CD45RA"),
trimodal_marker = c("CD4", "CD45RA"),
positive_peak = list(ADT = "CD3", sample = "buus_2021_T"),
save_fig = TRUE
)Case 2. Consider each healthy donor/patient per time point/condition/response/etc as one sample and normalize across the individual sample.
library(ADTnorm)
save_outpath <- "/path/to/output/location"
run_name <- "ADTnorm_demoRun"
data(cell_x_adt)
data(cell_x_feature)
cell_x_feature$batch = factor(cell_x_feature$study_name) ## consider each study as a batch
cell_x_adt_norm <- ADTnorm(
cell_x_adt = cell_x_adt,
cell_x_feature = cell_x_feature,
save_outpath = save_outpath,
study_name = run_name,
marker_to_process = c("CD3", "CD4", "CD8", "CD45RA"),
trimodal_marker = c("CD4", "CD45RA"),
positive_peak = list(ADT = "CD3", sample = "buus_2021_T"),
save_fig = TRUE
)Basic parameters introduction. The full parameter explanation for the ADTnorm function can be found at Reference - ADTnorm.
cell_x_adt: Matrix of ADT raw counts in cells (rows) by ADT markers (columns) format.
cell_x_feature: Matrix of cells (rows) by cell features (columns) such as sample, batch, and cell type-related information. Please note "sample" column is mandatory and should be the smallest unit to group the cells. At this resolution, ADTnorm will identify peaks and valleys to implement normalization. Please ensure the samples have different names across batches/conditions/studies. "batch" column is optional. It can be batches/conditions/studies/etc, that group the samples based on whether the samples are collected from the same batch run or experiment. This column is needed if the ```multi_sample_per_batch``` parameter is turned on to remove outlier positive peaks per batch or ```detect_outlier_valley``` for detecting and imputing outlier valleys per batch. If the "batch" column is not provided, it will be set as the same as the "sample" column. In the intermediate density plots that ADTnorm provides, density plots will be colored by the "batch" column.
save_outpath: The path to save the results.
study_name: Name of this run.
marker_to_process: Markers to normalize. Leave empty to process all the ADT markers in the cell_x_adt matrix.
bimodal_marker: Specify ADT markers that are likely to have two peaks based on researchers' prior knowledge or preliminary observation of the particular data to be processed. Leaving it as default, ADTnorm will try to find the bimodal peak in all markers that are not listed in `trimodal_marker.`
trimodal_marker: Index of the ADT markers that tend to have three peaks based on researchers' prior knowledge (e.g., CD4) or preliminary observation of the particular data to be processed.
positive_peak: A list variable containing a vector of ADT marker(s) and a corresponding vector of sample name(s) in matching order to specify that the uni-peak detected should be aligned to positive peaks. For example, for samples that only contain T cells, the only CD3 peak should be aligned to the positive peaks of other samples.
save_fig: Save the density plot figure for checking the peak and valley location detection.
For more detailed and typical parameter tuning examples, please visit tutorial website. We will illustrate using the demo data.
ADTnorm function will generate a matrix of rows of the same number as input cell_x_adt row number and columns are ADT markers specified in marker_to_process. The value in the matrix is normalized value by ADTnorm. In the save_outpath specified by the users, there will be two subfolders, figures and RDS, containing the intermediate object and density plot of detected peak and valley landmarks before and after ADTnorm. Those figures can be used to check whether certain ADT markers need further parameter tuning.
Case 2. Consider each healthy donor/patient per time point/condition/response/etc as one sample and normalize across the individual sample.
Color-coded by studies as batches.
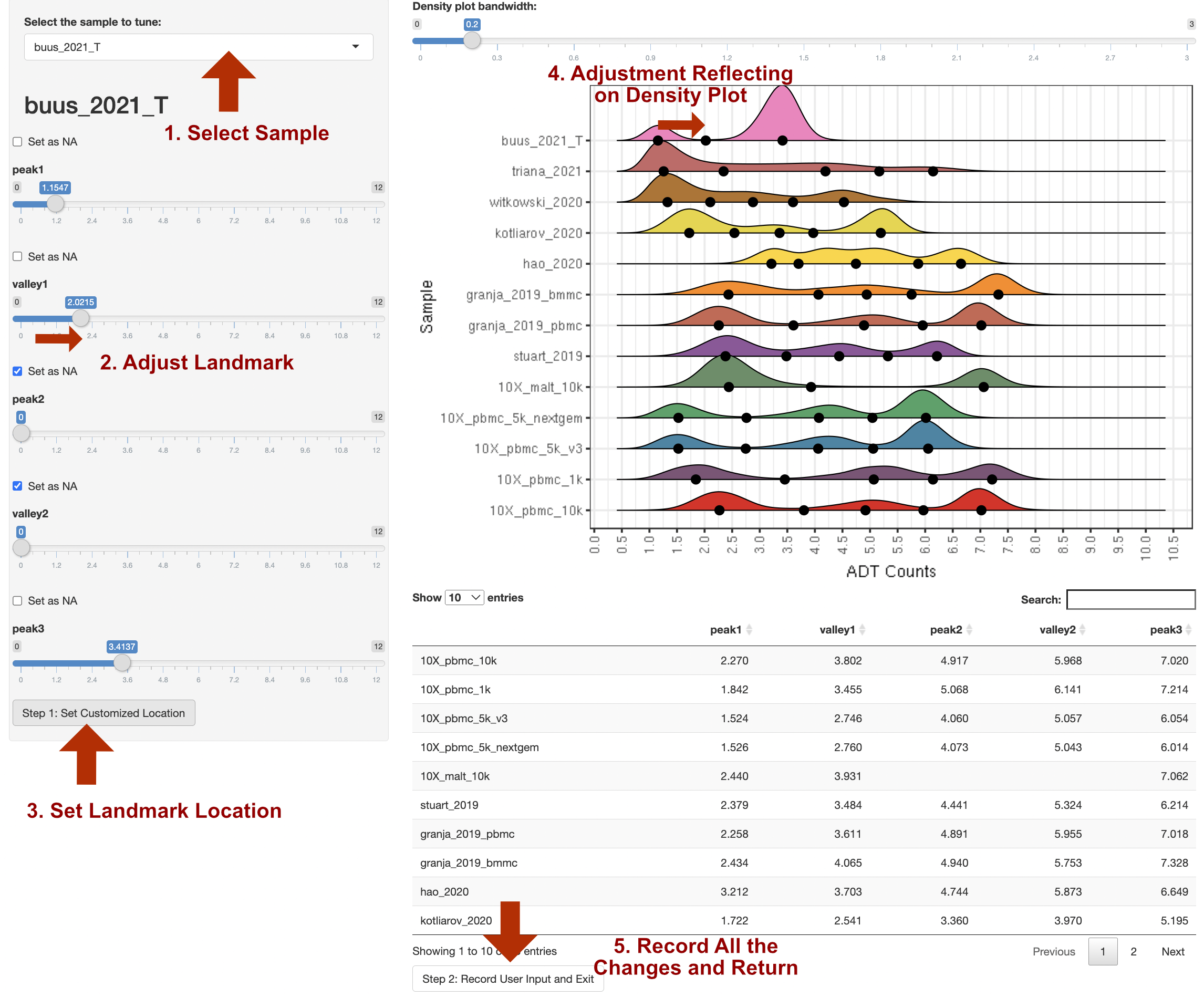
customize_landmark: By setting it to TRUE, ADTnorm will trigger the interactive landmark tuning function and pop out a shiny application for the user's manual setting of peak and valley locations. The procedure for adjusting the landmarks (peaks and valleys) is below.
Please note:
-
We recommend using this function after initial rounds of ADTnorm normalization with a few parameter tuning attempts. It is better to narrow down a few ADT markers that need manual tuning and provide the list to
marker_to_processas the interactive function will pop out for every marker being processed. -
If zigzag discrete negative peaks are observed, users can first increase the "Bandwidth for Density Visualization" at the top of the right panel to smooth out the discrete negative peaks before setting the landmarks.
-
Currently, the shiny browser support setting any landmark (peaks or valleys) to NA as missing. However, it does not support inserting new landmark(s). For example, if the marker density distribution shows a triple peak pattern but ADTnorm only detects two peaks across all the samples. Shiny browser does not allow manual insertion of a new peak and valley, but the user can tune the other parameters to push ADTnorm to detect three peaks: specify the target marker as
trimodal_marker, reducing thebw_smallest_trior setting smaller bandwidth value and specify for the target ADT marker throughbw_smallest_adjustments.
For more detailed and typical parameter tuning examples, please visit tutorial website. We will illustrate using the demo data.
Email: yzheng23@fredhutch.org
Twitter: @yezhengSTAT