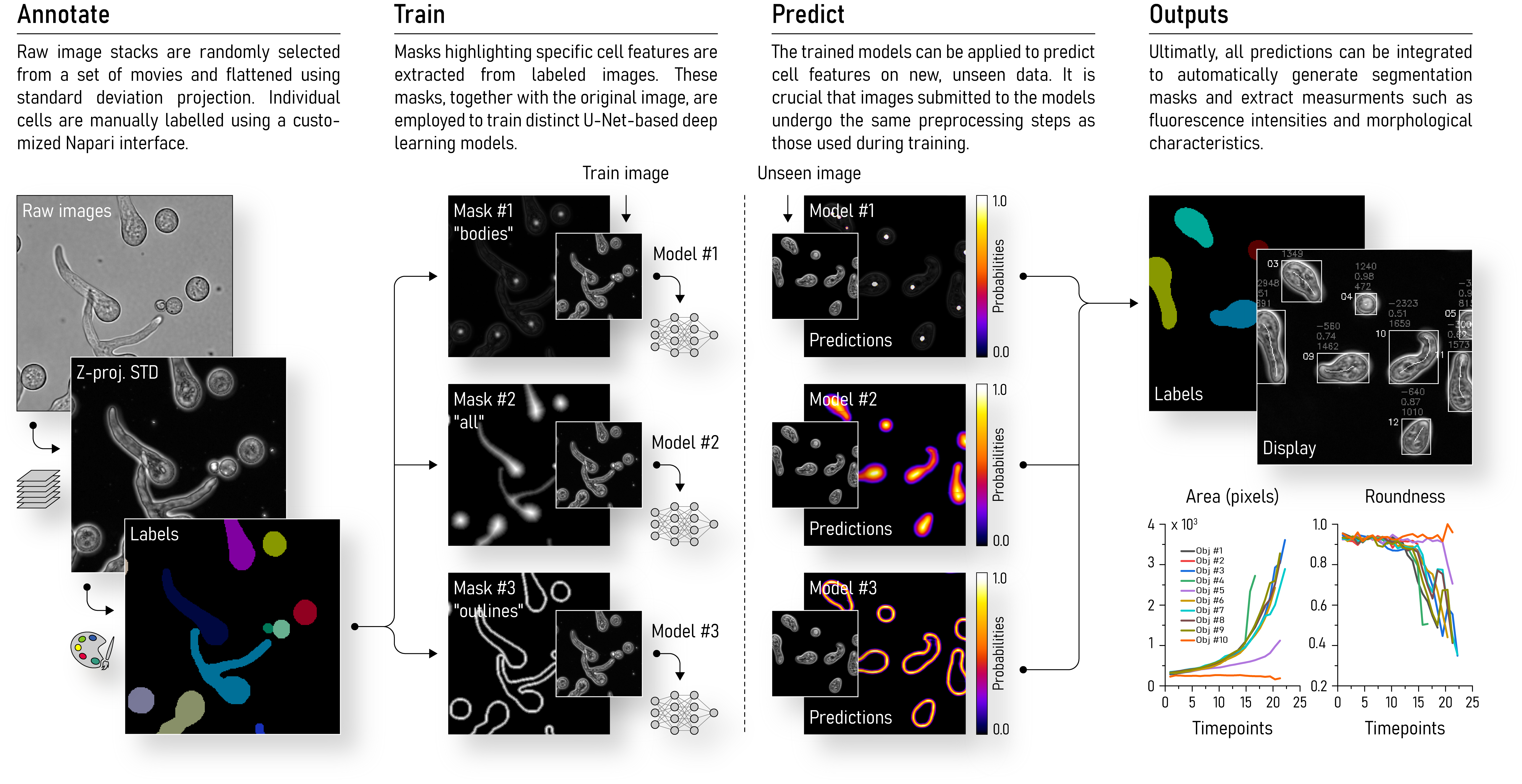
Spore germination analysis tool
Pease select your operating system
Windows
- Click on the green
<> Codebutton and downloadZIP - Unzip the downloaded file to a desired location
- Download and install Miniforge for your operating system
- Run the downloaded
.exefile- Select "Add Miniforge3 to PATH environment variable"
- Open the newly installed Miniforge Prompt
- Move to the downloaded GitHub repository
- Run one of the following command:
# TensorFlow with GPU support
mamba env create -f environment_tf_gpu.yml
# TensorFlow with no GPU support
mamba env create -f environment_tf_nogpu.yml- Activate Conda environment:
conda activate GasslerYour prompt should now start with (Gassler) instead of (base)
MacOS
- Click on the green
<> Codebutton and downloadZIP - Unzip the downloaded file to a desired location
- Download and install Miniforge for your operating system
- Open your terminal
- Move to the directory containing the Miniforge installer
- Run one of the following command:
# Intel-Series
bash Miniforge3-MacOSX-x86_64.sh
# M-Series
bash Miniforge3-MacOSX-arm64.sh- Re-open your terminal
- Move to the downloaded GitHub repository
- Run one of the following command:
# TensorFlow with GPU support
mamba env create -f environment_tf_gpu.yml
# TensorFlow with no GPU support
mamba env create -f environment_tf_nogpu.yml- Activate Conda environment:
conda activate GasslerYour prompt should now start with (Gassler) instead of (base)
Read data from remote_path, format and save to data_path
- Paths
- remote_path # str, path to remote directory
- data_path # str, path to saving directory
- exclude # list[str], exclude path(s) containing any of the str- Parameters
- pixSize_out # float, output pixel size (µm) Read data from data_path and execute the main procedure
- Paths
- remote_path # str, path to remote directory
- data_path # str, path to data directory
- target # str, "all" or "image_name"
- overwrite # bool, overwrite outputs- Parameters
- threshAll # float, threshold for "all" predictions
- threshOut # float, threshold for "outlines" predictions
- threshBod # float, threshold for "bodies" predictions
- min_size # int, min. size for tracked objects
- min_roundness # float, min. roundness for tracked objects- C1_proj.tif # channel 1 (spores) std-projection
- C2_proj.tif # channel 2 (bacteria) sum-projection
- display.tif # tracked objects display
- labels.tif # tracked objects labels
- composite.tif # C1_proj + C2_proj + display- data.pkl # PKL file containing all data
- area.csv # tracked objects areas
- intensity.csv # tracked objects C2 intensities
- length.csv # tracked objects length
- roundness.csv # tracked objects roundness
- plot.jpg # all data plot- Features
- Done
- Background subtraction for channel #2
- Measure object elongation
- Remove border touching objects
- Output CSV
- Save plots as JPG
- Object ID outputs & plots
- To do
- Remove early touching objects
- Manually reject objects from analysis
- Done
?logo=python&logoColor=rgb(149%2C157%2C165)&labelColor=rgb(50%2C60%2C65))
?logo=TensorFlow&logoColor=rgb(149%2C157%2C165)&labelColor=rgb(50%2C60%2C65))
?logo=NVIDIA&logoColor=rgb(149%2C157%2C165)&labelColor=rgb(50%2C60%2C65))
?logo=NVIDIA&logoColor=rgb(149%2C157%2C165)&labelColor=rgb(50%2C60%2C65))
&color=rgb(149%2C157%2C165))
&color=rgb(149%2C157%2C165))
&color=rgb(149%2C157%2C165))