- python 2.7.x (numpy, scipy, seaborn, pyBigWig, statsmodels, pysam)
- IEDB stand-alone (Note: IRIS is only tested on 20130222 2.15.5)
- bedtools 2.29.0
- STAR 2.5.3: required for IRIS RNA-seq processing
- samtools 1.3: required for IRIS RNA-seq processing
- rMATS-turbo: required for IRIS RNA-seq processing
- Cufflinks 2.2.1: required for IRIS RNA-seq processing
- seq2HLA: required for HLA typing (Note: The original URL of the tool is no longer working); requires bowtie
- MS GF+ (v2018.07.17): required for MS search; requiring Java
- R: used by seq2HLA
The IRIS program can be downloaded directly from the repository, as shown below:
git clone https://github.com/Xinglab/IRIS.git
cd IRIS
IRIS is designed to make use of a computing cluster to improve performance. For users who want to enable cluster execution for functions that support it (see Configure for details), please update the contents of snakemake_profile/ to ensure compatibility with the available compute environment.
IRIS loads a big-data reference database of splicing events and other genomic annotations. These data are included in IRIS_data.v2.0.0 (a Google Drive link; size of entire folder is ~400 GB; users can select reference groups to download). The files need to be placed under ./IRIS_data/
The files can be automatically downloaded with google_drive_download.py. Downloading a large amount of data with the API requires authentication:
- https://cloud.google.com/docs/authentication/production
- https://cloud.google.com/bigquery/docs/authentication/service-account-file
To use the script, first create a service account:
- Go to google cloud console -> IAM & Admin -> Service Accounts -> create service account
- Give the new account: role=owner
- Click the new service account email on the service account page
- Download a .json key by clicking: keys -> add key -> create new key -> json
That .json key is passed to google_drive_download.py
Download IEDB_MHC_I-2.15.5.tar.gz from the IEDB website (see Dependencies). Create a folder named IEDB/ in the IRIS folder, then move the downloaded gz file to IEDB/. From http://tools.iedb.org/main/download/
- click "MHC Class I"
- click "previous version"
- find and download version 2.15.5
The manual download is needed because there is a license that must be accepted.
./install can automatically install most dependencies to conda environments:
- conda must already be installed for the script to work
- The install script will check if
IRIS_data/has been downloaded- To download see 1.2 Download IRIS db
- The install script will check if IEDB tools has been downloaded
- To download see 1.3 Download IEDB MHC I prediction tools
Under the IRIS folder, to install IRIS core dependencies, do:
./install core
To install optional dependencies not needed for the most common IRIS usage:
./install all
Snakefile describes the IRIS pipeline. The configuration for running jobs can be set by editing snakemake_profile/. The provided configuration adapts IRIS to use Slurm. Other compute environments can be supported by updating this directory
- snakemake_profile/config.yaml: Sets various Snakemake parameters including whether to submit jobs to a cluster.
- snakemake_profile/cluster_submit.py: Script to submit jobs.
- snakemake_profile/cluster_status.py: Script to check job status.
- snakemake_profile/cluster_commands.py: Commands specific to the cluster management system being used. The default implementation is for Slurm. Other cluster environments can be used by changing this file. For example, snakemake_profile/cluster_commands_sge.py can be used to overwrite
cluster_commands.pyto support an SGE cluster. - To force Snakemake to execute on the local machine modify snakemake_profile/config.yaml:
- comment out
cluster - set
jobs: {local cores to use} - uncomment the
resourcessection and setmem_mb: {MB of RAM to use}
- comment out
- The conda install of Python 2 may give an error like
ImportError: No module named _sysconfigdata_x86_64_conda_linux_gnu- Check for the error by activating
conda_env_2and runningpython - Resolve with commands similar to
cd conda_env_2/lib/python2.7/cp _sysconfigdata_x86_64_conda_cos6_linux_gnu.py _sysconfigdata_x86_64_conda_linux_gnu.py
- Check for the error by activating
- IRIS uses
--label-stringto determine which fastq files are for read 1 and read 2- To avoid any issues name your fastq files so that they end with
1.fastqand2.fastqto indicate which file represents which pair of the read
- To avoid any issues name your fastq files so that they end with
- For streamlined AS-derived target discovery, please follow major functions and run the corresponding toy example.
- For customized pipeline development, please check all functions of IRIS.
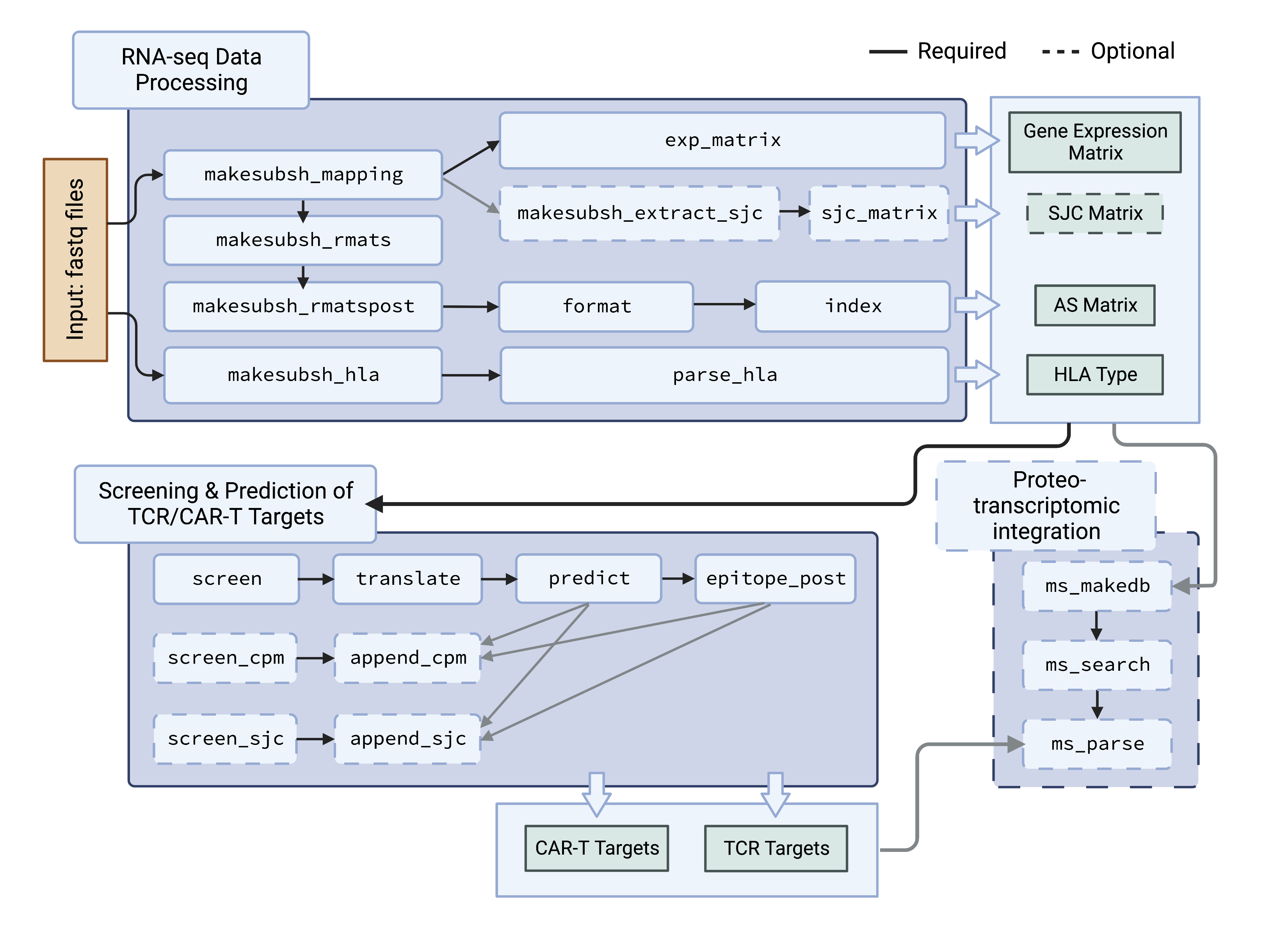
This flowchart shows how the IRIS functions are organized
IRIS provides individual functions/steps, allowing users to build pipelines for their customized needs. IRIS_functions.md describes each model/step, including RNA-seq preprocessing, HLA typing, proteo-transcriptomic MS searching, visualization, etc.
usage: IRIS [-h] [--version]
positional arguments:
{format,screen,predict,epitope_post,process_rnaseq,makesubsh_mapping,makesubsh_rmats,makesubsh_rmatspost,exp_matrix,makesubsh_extract_sjc,extract_sjc,sjc_matrix,index,translate,pep2epitope,screen_plot,screen_sjc,append_sjc,annotate_ijc,screen_cpm,append_cpm,screen_novelss,screen_sjc_plot,makesubsh_hla,parse_hla,ms_makedb,ms_search,ms_parse,visual_summary}
format Format AS matrices from rMATS, followed by indexing
for IRIS
screen Identify AS events of varying degrees of tumor
association and specificity using an AS reference
panel
predict Predict and annotate AS-derived TCR (pre-prediction)
and CAR-T targets
epitope_post Post-prediction step to summarize predicted TCR
targets
process_rnaseq Process RNA-Seq FASTQ files to quantify gene
expression and AS
makesubsh_mapping Make submission shell scripts for running
'process_rnaseq'
makesubsh_rmats Makes submission shell scripts for running rMATS-turbo
'prep' step
makesubsh_rmatspost
Make submission shell scripts for running rMATS-turbo
'post' step
exp_matrix Make a merged gene expression matrix from multiple
cufflinks results
makesubsh_extract_sjc
Make submission shell scripts for running
'extract_sjc'
extract_sjc Extract SJ counts from STAR-aligned BAM file and
annotates SJs with number of uniquely mapped reads
that support the splice junction.
sjc_matrix Make SJ count matrix by merging SJ count files from a
specified list of samples. Performs indexing of the
merged file
index Index AS matrices for IRIS
translate Translate AS junctions into junction peptides
pep2epitope Wrapper to run IEDB for peptide-HLA binding prediction
screen_plot Make stacked/individual violin plots for list of AS
events
screen_sjc Identify AS events of varying degrees of tumor
specificity by comparing the presense-absense of
splice junctions using a reference of SJ counts
append_sjc Append "screen_sjc" result as an annotation to PSI-
based screening results and epitope prediction results
in a specified screening output folder
annotate_ijc Annotate inclusion junction count info to PSI-based
screening results or epitope prediction results in a
specified screening output folder. Can be called from
append_sjc to save time
screen_cpm Identify AS events of varying degrees of tumor
association and specificity using an AS reference
panel based on normalized splice junction read counts
append_cpm Append "screen_cpm" result as an annotation to PSI-
based screening results and epitope prediction results
in a specified screening output folder
screen_novelss Identify AS events of varying degrees of tumor
association and specificity, including events with
unannotated splice junctions deteced by rMATS
"novelss" option
screen_sjc_plot Make stacked/individual barplots of percentage of
samples expressing a splice junction for list of AS
events
makesubsh_hla Make submission shell scripts for running seq2HLA for
HLA typing using RNA-Seq
parse_hla Summarize seq2HLA results of all input samples into
matrices for IRIS use
ms_makedb Generate proteo-transcriptomic database for MS search
ms_search Wrapper to run MSGF+ for MS search
ms_parse Parse MS search results to generate tables of
identified peptides
visual_summary Make a graphic summary of IRIS results
optional arguments:
-h, --help show this help message and exit
--version show program's version number and exit
For command line options of each sub-command, type: IRIS COMMAND -h
The core of IRIS immunotherapy target discovery comprises of four steps from three major modules. For a quick test, see Example which uses the snakemake to run a small data set.
- Step 1. Generate and index PSI-based AS matrix from rMATS output (RNA-seq data processing module)
IRIS formatoption-dshould be used to save the generated PSI-based AS matrix to the downloaded IRIS DB.- Example files for
rmats_mat_path_manifestandrmats_sample_ordercan be found under example/example_of_input_to_format_step/. IRIS indexwill create an index for the IRIS format generated PSI-based AS matrix, and-oshould be the path to the folder containing the generated AS matrix.- The option
novelSSis experimental and not fully validated. It takes the output from the experimental function in rMATS to identify events with unannotated splice sites. Please refer to the latest rMATS (> v4.0.0) for details.
usage: IRIS format [-h] -t {SE,RI,A3SS,A5SS} -n DATA_NAME -s {1,2}
[-c COV_CUTOFF] [-i] [-e] [-d IRIS_DB_PATH] [--novelSS]
[--gtf GTF]
rmats_mat_path_manifest rmats_sample_order
usage: IRIS index [-h] -t {SE,RI,A3SS,A5SS} -n DATA_NAME
[-c COV_CUTOFF] [-o OUTDIR]
splicing_matrix
- Step 2. Screen and translate tumor-associated events (IRIS screening module: 'tumor-association screen' + optional 'tumor-recurrence screen')
- Description of the
PARAMETER_FINinput file can be found at example/parameter_file_description.txt, and an example file can be found at example/NEPC_test.para. - To perform an optional tumor-recurrence screen, include a 'tumor reference' in the
PARAMETER_FINinput file. - Users can also use an optional secondary tumor-association screen (not included in Snakemake) by calling
IRIS screen_cpm. This screening test accounts for the joint effects of overall gene expression and AS. Commands to run this test and the output result format are similar to tumor-specificity test in the 'Step 4' below. - Option
-tinIRIS screenrunsIRIS translateto generate SJ peptides, a required step for IRIS module for target prediction.
- Description of the
usage: IRIS screen [-h] -p PARAMETER_FIN
--splicing-event-type {SE,RI,A3SS,A5SS} -o OUTDIR [-t]
[-g GTF] [--all-reading-frames] [--ignore-annotation]
[--remove-early-stop] [--min-sample-count MIN_SAMPLE_COUNT]
[--use-existing-test-result]
usage: IRIS translate [-h] -g REF_GENOME
--splicing-event-type {SE,RI,A3SS,A5SS} --gtf GTF
-o OUTDIR [--all-orf] [--ignore-annotation]
[--remove-early-stop] [-c DELTAPSI_COLUMN] [-d DELTAPSI_CUT_OFF]
[--no-tumor-form-selection] [--check-novel]
as_input
- Step 3. Predict both extracellular targets and epitopes(designed for cluster execution) (IRIS target prediction module)
IRIS predictcan generate CAR-T annotation results and prepare a job array submission for TCR epitope prediction. TCR prediction preparation is optional and can be disabled by using--extraceullular-only.IRIS epitope_postwill summarize TCR epitope prediction results after TCR epitope prediction jobs from IRIS predict are submitted and finished (job array submission step can be done manually or using snakemake)MHC_LISTandMHC_BY_SAMPLEcan be generated by runningHLA_typing(within or outside of IRIS). Note that it is not necessary to restrict HLA types detected from input RNA samples. It is recommended for users to specify dummy files only containing HLA types of interest or common HLA types as long as HLA types in the dummyhla_types.listandhla_patient.tsvare consistent. Example files forhla_types.listandhla_patient.tsvcan be found at example/hla_types_test.list and example/hla_patient_test.tsv respectively.
usage: IRIS predict [-h] --task-dir TASK_DIR -p PARAMETER_FIN
-t {SE,RI,A3SS,A5SS} [--iedb-local IEDB_LOCAL]
[-m MHC_LIST] [--extracellular-only] [--tier3-only]
[--gene-exp-matrix GENE_EXP_MATRIX] [-c DELTAPSI_COLUMN]
[-d DELTAPSI_CUT_OFF] [-e EPITOPE_LEN_LIST] [--all-reading-frames]
[--extracellular-anno-by-junction]
IRIS_screening_result_path
usage: IRIS epitope_post [-h] -p PARAMETER_FIN -o OUTDIR
-t {SE,RI,A3SS,A5SS} -m MHC_BY_SAMPLE
-e GENE_EXP_MATRIX [--tier3-only] [--keep-exist]
[--epitope-len-list EPITOPE_LEN_LIST]
[--no-match-to-canonical-proteome]
[--no-uniqueness-annotation]
[--ic50-cut-off IC50_CUT_OFF]
- Step 4. Perform tumor-specificity screen, a more strigent screen comparing the presence-absence of a given SJ between tumor and normal tissues (IRIS screening module: 'tumor-specificity screen')
IRIS append_sjccombinesscreenandscreen_sjcresults (by appendingscreen_sjcoutputs toscreenoutputs). This 'integrated' output contains annotations for tumor-specific targets.IRIS append_sjc -ioption can be used to execute bothIRIS append_sjcandIRIS annotate_ijcfunctions. If-ioption is used,-pand-earguments are required.
usage: IRIS screen_sjc [-h] -p PARAMETER_FIN
--splicing-event-type {SE,RI,A3SS,A5SS}
-e EVENT_LIST_FILE -o OUTDIR
[--use-existing-test-result]
[--tumor-read-cov-cutoff TUMOR_READ_COV_CUTOFF]
[--normal-read-cov-cutoff NORMAL_READ_COV_CUTOFF]
usage: IRIS append_sjc [-h] --sjc-summary SJC_SUMMARY
--splicing-event-type {SE,RI,A3SS,A5SS} -o OUTDIR
[-i] [-u] [-p PARAMETER_FILE]
[-e SCREENING_RESULT_EVENT_LIST]
[--inc-read-cov-cutoff INC_READ_COV_CUTOFF]
[--event-read-cov-cutoff EVENT_READ_COV_CUTOFF]
usage: IRIS annotate_ijc [-h] -p PARAMETER_FILE
--splicing-event-type {SE,RI,A3SS,A5SS}
-e SCREENING_RESULT_EVENT_LIST -o OUTDIR
[--inc-read-cov-cutoff INC_READ_COV_CUTOFF]
[--event-read-cov-cutoff EVENT_READ_COV_CUTOFF]
The Snakemake workflow can be run with ./run. First set the configuration values in snakemake_config.yaml:
- Set the resources to allocate for each job:
{job_name}_{threads}{job_name}_{mem_gb}{job_name}_{time_hr}
- Set the reference files:
- Provide the file names for
gtf_name:andfasta_name: - Either place the files in
./references/or provide a URL underreference_files:to download the (potentially gzipped) files:
- Provide the file names for
gtf_name: 'some_filename.gtf'
fasta_name: 'other_filename.fasta'
reference_files:
some_filename.gtf.gz:
url: 'protocol://url/for/some_filename.gtf.gz'
other_filename.fasta.gz:
url: 'protocol://url/for/other_filename.fasta.gz'
- Set the input files:
sample_fastqs:Set the read 1 and read 2 fastq files for each sample. For example:
sample_fastqs:
sample_name_1:
- '/path/to/sample_1_read_1.fq'
- '/path/to/sample_1_read_2.fq'
sample_name_2:
- '/path/to/sample_2_read_1.fq'
- '/path/to/sample_2_read_2.fq'
blocklist: an optional blocklist of AS events similar to IRIS/data/blocklist.brain_2020.txtmapability_bigwig: an optional file for evaluating splice region mappability similar toIRIS_data/resources/mappability/wgEncodeCrgMapabilityAlign24mer.bigWigmhc_list: required if not starting with fastq files, similar to example/hla_types_test.listmhc_by_sample: required if not starting with fastq files, similar to example/hla_patient_test.tsvgene_exp_matrix: optional tsv file with geneName as the first column and the expression for each sample in the remaining columnssplice_matrix_txt: optional output file from IRIS index that can be used as a starting pointsplice_matrix_idx: the index file forsplice_matrix_txtsjc_count_txt: optional output file fromIRIS sjc_matrixthat can be used as a starting point. Only relevant ifshould_run_sjc_stepssjc_count_idx: the index file forsjc_count_txt
- Set other options
run_core_modules: set totrueto start with existingIRIS formatoutput and HLA listsrun_all_modules: set totrueto start with fastq filesshould_run_sjc_steps: set totrueto enable splice junction based evaluation stepsstar_sjdb_overhang: used by STAR alignment. Should ideally beread_length -1, but the STAR manual says that 100 works well as a defaultrun_name: used to name output files that will be written toIRIS_data/splice_event_type: one of[SE, RI, A3SS, A5SS]comparison_mode: one of[group, individual]stat_test_type: one of[parametric, nonparametric]use_ratio: set totrueto require a ratio of reference groups to pass the checks rather than a fixed counttissue_matched_normal_..._{cutoff}: set cutoffs for the tissue matched normal reference group (tier 1)tissue_matched_normal_reference_group_names: a comma separated list of directory names underIRIS_data/dbtumor_..._{cutoff}: set cutoffs for the tumor reference group (tier 2)tumor_reference_group_names: a comma separated list of directory names underIRIS_data/dbnormal_..._{cutoff}: set cutoffs for the normal reference group (tier 3)normal_reference_group_names: a comma separated list of directory names underIRIS_data/db
The snakemake is configured to run the above IRIS streamlined major functions using example/. For customized pipeline development, we recommend that users refer to the Snakefile as a reference. Snakefile defines the steps of the pipeline. To run the example, update the /path/to/ values with full paths in snakemake_config.yaml, and make any adjustments to snakemake_profile/. Then
./run
As mentioned in Usage, the full example is designed to be run with a compute cluster. It will take < 5 min for the formatting and screening steps and usually < 15 min for the prediction step (depending on available cluster resources).
A successful test run will generate the following result files in ./results/NEPC_test/screen/ (row numbers are displayed before each file name):
0 NEPC_test.SE.notest.txt
1 NEPC_test.SE.test.all_guided.txt
1 NEPC_test.SE.tier1.txt
1 NEPC_test.SE.tier1.txt.integratedSJC.txt
4 NEPC_test.SE.tier2tier3.txt.ExtraCellularAS.txt
4 NEPC_test.SE.tier2tier3.txt.ExtraCellularAS.txt.integratedSJC.txt
6 NEPC_test.SE.tier2tier3.txt
6 NEPC_test.SE.tier2tier3.txt.ijc_info.txt
6 NEPC_test.SE.tier2tier3.txt.integratedSJC.txt
11 NEPC_test.SE.test.all_voted.txt
4 SE.tier2tier3/epitope_summary.junction-based.txt
4 SE.tier2tier3/epitope_summary.junction-based.txt.integratedSJC.txt
9 SE.tier2tier3/epitope_summary.peptide-based.txt
9 SE.tier2tier3/epitope_summary.peptide-based.txt.integratedSJC.txt
11 SE.tier2tier3/pred_filtered.score500.txt
A summary graphic is generated to ./results/NEPC_test/visualization/summary.png
Final reports are shown in bold font.
[TASK/DATA_NAME].[AS_TYPE].test.all_guided.txt: All AS events tested by IRIS screening with tissue-matched normal tissue reference panel available. One-sided test will be used to generate p-value.
[TASK/DATA_NAME].[AS_TYPE].test.all_voted.txt: All AS events tested by IRIS screening without tissue-matched normal tissue reference panel. Two-sided test will be used to generate p-value for comparisons to normal panels.
[TASK/DATA_NAME].[AS_TYPE].notest.txt: During screening, AS events skipped due to no variance or no available comparisons
[TASK/DATA_NAME].[AS_TYPE].tier1.txt: Tumor-associated AS events after comparison to tissue-matched normal panel ('tier1' events)
[TASK/DATA_NAME].[AS_TYPE].tier2tier3.txt: Tumor-associated AS events after comparison to tissue-matched normal panel, tumor panel, and normal tissue panel ('tier3' AS events)
[TASK/DATA_NAME].[AS_TYPE].tier1.txt.ExtraCellularAS.txt: Tumor-associated AS events in 'tier1' set that are associated with protein extracellular annotation and may be used for CAR-T targets
[TASK/DATA_NAME].[AS_TYPE].tier2tier3.txt.ExtraCellularAS.txt: Tumor-associated AS events in 'tier3' set that are associated with protein extracellular annotation and may be used for CAR-T targets
[AS_TYPE].tier1/pred_filtered.score500.txt: IEDB prediction outputs for SJ peptides from 'tier1' set with HLA-peptide binding IC50 values passing user-defined cut-off
[AS_TYPE].tier1/epitope_summary.peptide-based.txt: AS-derived epitopes from 'tier1' set that are predicted to bind user-defined HLA types
[AS_TYPE].tier1/epitope_summary.junction-based.txt: AS events from 'tier1' set that are predicted to bind user-defined HLA types
[AS_TYPE].tier2tier3/pred_filtered.score500.txt: IEDB prediction outputs for AS junction peptides from 'tier3' set with HLA-peptide binding IC50 value passing user-defined cut-off
[AS_TYPE].tier2tier3/epitope_summary.peptide-based.txt: AS-derived epitopes from 'tier3' set that are predicted to bind user-defined HLA types
[AS_TYPE].tier2tier3/epitope_summary.junction-based.txt: AS events from 'tier3' set that are predicted to bind user-defined HLA types
Screening or prediction outputs that integrate screen and screen_sjc results contain annotations for tumor-specific targets. These output files are indicated by .integratedSJC.txt, such as [TASK/DATA_NAME].[AS_TYPE]tier2tier3.txt.integratedSJC.txt and [AS_TYPE].tier2tier3/epitope_summary.peptide-based.txt.integratedSJC.txt, etc.
Yang Pan panyang@ucla.edu
Eric Kutschera KUTSCHERAE@chop.edu
Beatrice Zhang beazhang@sas.upenn.edu
Yi Xing yxing@ucla.edu
Pan Y*, Phillips JW*, Zhang BD*, Noguchi M*, Kutschera E, McLaughlin J, Nesterenko PA, Mao Z, Bangayan NJ, Wang R, Tran W, Yang HT, Wang Y, Xu Y, Obusan MB, Cheng D, Lee AH, Kadash-Edmondson KE, Champhekar A, Puig-Saus C, Ribas A, Prins RM, Seet CS, Crooks GM, Witte ON+, Xing Y+. (2023) IRIS: Big data-informed discovery of cancer immunotherapy targets arising from pre-mRNA alternative splicing. PNAS, in press. (+joint corresponding authors; *joint first authors)