This repository contains a collection of bash and R scripts that were
developped to analyze Chromosome Conformation Capture on Chip (4C) data,
meaning the microarray version of 4C which was employed in early genomic
studies of chromosome conformations.
The data processing workflow is built upon improved versions of the procedures
we used in Bantignies et al. 2011, with methods addressing the selection
and normalization of microarray probes, and the multi-resolution visualization
and segmentation of 4C profiles, which are directly accessible in the standalone
R package MRA.TA.
A detailed presentation of these methods as well as a brief introduction to the microarray version of the 4C technique can be found in Leblanc et al. 2016. To put this in the perspective of Chromosome Conformation Capture (3C) methods and applications, one can for instance read the review from Denker & de Laat 2016.
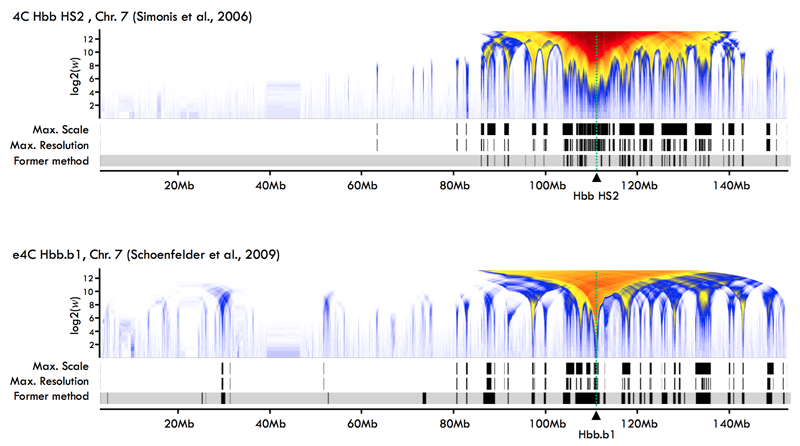
The figure below shows results produced using the MiMB.4C workflow
with 4C data in mouse from Simonis et al. 2006 (top panel) and
Schoenfelder et al. 2009 (bottom panel).
Each panel represents the mouse chromosome 7 on the horizontal axis and the resolution of analysis on the vertical axis, in number of microarray probes (w). In both studies the 4C "bait" or "anchor" sequence was targeting the beta globin locus (Hbb, dotted green line).
The frequencies of interactions between the Hbb locus and remote locations along the chromosome are indicated by colors, from light blue for the weakest to dark red for the strongest ones, representing the multi-resolution statistics proposed in de Wit et al. 2008.
The 3 tracks below each colormap show alternative segmentations of the most significant interactions, indicating from top to bottom:
- a segmentation at maximal scale and at maximal resolution resulting from our workflow.
- the segmentation reported in the original study using former data analysis methods.
The following sections describe how to run the MiMB.4C workflow using 4C data
in Drosophila anterior larval tissues from Bantignies et al. 2011.
- Run the
Rcode below to install the MRA.TA package.
If the installation fails, try to install dependencies manually as indicated in the next section.
library("devtools")
install_github("benja0x40/MRA.TA")-
Clone or download and decompress the
MiMB.4Crepository. -
Run the demo in a terminal (
bash), with current working directory at the root of the decompressedMiMB.4Cfolder.
./dataPreparation.sh
Rscript enrichmentAnalysis.Rbowtieshort read aligner version 1.x (http://bowtie-bio.sourceforge.net)- R environment version 3.x
- CRAN packages
devtools,stringr,getopt,plotrix - Bioconductor packages
Biostrings,GenomicRanges - GitHub
Rpackage MRA.TA
Run the R code below to install CRAN, Bioconductor and GitHub package
dependencies for MiMB.4C.
# Already installed
pkg <- installed.packages()[, "Package"]
# CRAN packages
lst <- c("devtools", "stringr", "getopt", "plotrix")
lst <- setdiff(lst, pkg)
if(length(lst) > 0) install.packages(lst, repos = "https://cloud.r-project.org/")
# Bioconductor packages
lst <- c("Biostrings", "GenomicRanges")
lst <- setdiff(lst, pkg)
if(length(lst) > 0) {
source("https://bioconductor.org/biocLite.R")
biocLite(lst)
}
# GitHub package
library("devtools")
install_github("benja0x40/MRA.TA")-
importGenome.shTool to download a genome sequence from UCSC and create its
bowtieindex. -
importRawData.shTool to download the demo 4C data from Gene Expression Omnibus (GEO).
-
dataPreparation.shWorkflow script to be executed first when running the demo analysis. This script chains several operations: importing genome sequence and raw data, updating micro array design probes to the lastest (dm6) genome assembly, filtering out non-experimental probes, mapping bait sequence (Fab7) to the genome, and computing the restriction map associated to the 4C protocol (DpnII).
-
updateDesignData.RTool to update micro array design information for any genome release. This tool uses
bowtieto map probe sequences on a chosen genome assembly and updates genomic coordinates addressed by the microarray platform accordingly. -
computeRestrictionMap.RTool to compute the genomic coordinates of all restriction sites for a given restriction enzyme. Restriction sites are defined by the short DNA motif, commonly 4 to 6bp in 4C protocols, specifically targeted by the enzyme.
-
enrichmentAnalysis.RWorkflow script to be executed secondly (after
dataPreparation.sh) when running the demo analysis. This script chains normalization, probes filtering and multi-resolution analysis of the 4C enrichments.For further details see Leblanc et al. 2016.
4C_Bait_Sequences => Fasta file of 4C bait sequence and its bowtie alignments
Experiment_Design => Tables defining necessary 4C experiment data annotations
Genome_Data => UCSC genome data and corresponding bowtie indexes
UCSC_dm6 => dm6 assembly of the Drosophila melanogaster genome
Raw_Data => Raw 4C data
GSE23887_RAW => 4C data from Bantignies et al. 2011
Processed_Data => Pre-processed 4C data
RestrictionMap => Coordinates of restriction fragments
Updated => Updated microarray design data (new probes coordinates)
Cleaned => Updated array design filtered out for non-relevant probes
Runinng importGenome.sh or importRawData.sh bash scripts without any
parameters will show information about available parameters.
The standalone R scripts updateDesignData.R and computeRestrictionMap.R can
also provide help on available parameters by using the option -h.
For instance, using the terminal:
./importGenome.sh
Rscript updateDesignData.R -h
Rscript computeRestrictionMap.R -hThanks to Elzo de Wit for kindly sharing his source code and suggestions on the multi-resolution methods, and to Bas Tolhuis who greatly helped with Nimblegen tiling array data analyses, also sharing source code as well as unpublished biological data. Thanks to Jean-Philippe Villemin for testing the installation and execution of the complete workflow and reporting issues and suggestions.
1. Bantignies F., Roure V., Comet I., Leblanc B., Schuettengruber B., Bonnet J., Tixier V., Mas A., Cavalli G. Polycomb-dependent regulatory contacts between distant Hox loci in Drosophila. Cell (2011).
publisher | pubmed
2. Leblanc B., Comet I., Bantignies F., and Cavalli G., Chromosome Conformation Capture on Chip (4C): data processing. Book chapter in Polycomb Group Proteins: Methods and Protocols. Lanzuolo C., Bodega B. editors, Methods in Molecular Biology (2016).
publisher | pubmed
3. Denker A. & de Laat W., The second decade of 3C technologies: detailed insights into nuclear organization. Genes & Development (2016).
publisher | pubmed
4. Simonis M., Klous P., Splinter E., Moshkin Y., Willemsen R., de Wit E., van Steensel B., de Laat W. Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture-on-chip (4C). Nature Genetics (2006).
publisher | pubmed
5. Schoenfelder S., Sexton T., Chakalova L., Cope N.F., Horton A., Andrews S., Kurukuti S., Mitchell J.A., Umlauf D., Dimitrova D.S., Eskiw C.H., Luo Y., Wei C.L., Ruan Y., Bieker J.J, Fraser P. Preferential associations between co-regulated genes reveal a transcriptional interactome in erythroid cells. Nature Genetics (2009).
publisher | pubmed
6. de Wit E., Braunschweig U., Greil F., Bussemaker H. J. & van Steensel B. Global chromatin domain organization of the Drosophila genome. PLoS Genetics (2008).
publisher | pubmed