circRNA quantification, differential expression analysis and miRNA target prediction of RNA-Seq data.
nf-core/circrna is a best-practice analysis pipeline for the quantification, miRNA target prediction and differential expression analysis of circular RNAs in paired-end RNA sequencing data.
The pipeline is built using Nextflow, a workflow tool to run tasks across multiple compute infrastructures in a very portable manner. It comes with docker containers making installation trivial and results highly reproducible.
-
Install
Nextflow(>=21.04.0) -
Install any of
Docker,Singularity,Podman,ShifterorCharliecloudfor full pipeline reproducibility (please only useCondaas a last resort; see docs) -
Download the pipeline and test it on a minimal dataset with a single command:
nextflow run nf-core/circrna -profile test,<docker/singularity/podman/shifter/charliecloud/conda/institute>
Please check nf-core/configs to see if a custom config file to run nf-core pipelines already exists for your Institute. If so, you can simply use
-profile <institute>in your command. This will enable eitherdockerorsingularityand set the appropriate execution settings for your local compute environment. -
Start running your own analysis!
nextflow run nf-core/circrna -profile <docker/singularity/podman/shifter/charliecloud/conda/institute> --module 'circrna_discovery, mirna_prediction, differential_expression' --tool 'circexplorer2' --input 'samples.csv' --input_type 'fastq' --phenotype 'phenotype.csv'
Refer to usage documentation for exapanded details on running each analysis module.
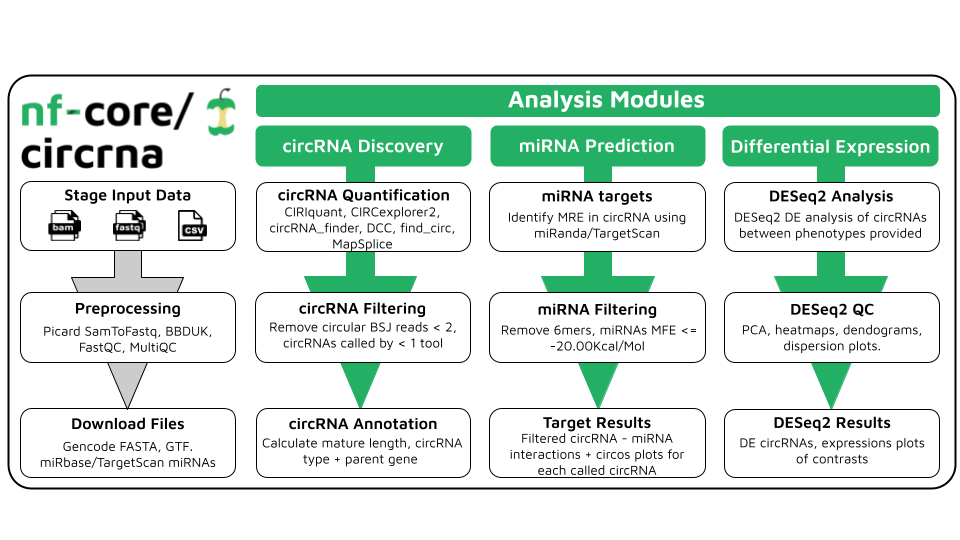
- Input type conversion
SamToFastq - Raw read quality control
FastQC - Adapter trimming + read filtering
BBDUK - circRNA quantification
- circRNA filtering
- Filter candidate circRNAs by number of reads spanning back-splice junction
- circRNA annotation
- Annotate candidates as circRNA, ciRNA or EI-circRNA
- Calculate mature spliced length
- Export mature spliced length as FASTA file
- Annotate parent gene, underlying transcripts.
- Export information as customised BED12 file
- circRNA count matrix
- Combine results of quantification tools to produce counts matrix for downstream statistical analysis
- Require circRNAs in matrix to be called by at least n quantification tools (consensus filtering)
- miRNA target prediction
miRandaTargetScan- Filter results, miRNAs must be called by both tools
- Differential expression analysis
DESeq2 - MultiQC report
MultiQC
Ouputs given by each step in the pipeline can be viewed in the output documentation
The nf-core/circrna pipeline comes with documentation about the pipeline: usage and output.
nf-core/circrna was originally written by Barry Digby (@BarryDigby) from the National University of Ireland, Galway as a member of Dr. Pilib Ó Broins lab with the financial support of Science Foundation Ireland (Grant number 18/CRT/6214).
If you would like to contribute to this pipeline, please see the contributing guidelines.
For further information or help, don't hesitate to get in touch on the Slack #circrna channel (you can join with this invite).
You can cite the nf-core publication as follows:
The nf-core framework for community-curated bioinformatics pipelines.
Philip Ewels, Alexander Peltzer, Sven Fillinger, Harshil Patel, Johannes Alneberg, Andreas Wilm, Maxime Ulysse Garcia, Paolo Di Tommaso & Sven Nahnsen.
Nat Biotechnol. 2020 Feb 13. doi: 10.1038/s41587-020-0439-x.
In addition, references of tools and data used in this pipeline are as follows:
- BBDUK Bushnell, B. (Unpublished). Download: https://sourceforge.net/projects/bbmap/
- bedtools Quinlan, A.R. & Hall, I.M., (2010). BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics , 26(6), pp.841–842. Available at: http://dx.doi.org/10.1093/bioinformatics/btq033. Download: https://github.com/arq5x/bedtools2/releases
- Bowite Langmead, B., Trapnell, C., Pop, M. et al., (2009). Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10, R25. Availabe at: https://doi.org/10.1186/gb-2009-10-3-r25. Download: https://sourceforge.net/projects/bowtie-bio/
- Bowtie2 Langmead, B. & Salzberg, S. L. (2012). Fast gapped-read alignment with Bowtie 2. Nature methods, 9(4), p. 357–359. Available at: 10.1038/nmeth.1923. Download: http://bowtie-bio.sourceforge.net/bowtie2/index.shtml
- bwa Li, H., & Durbin, R. (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics , 25(14), 1754–1760. Available at: https://doi.org/10.1093/bioinformatics/btp324. Download: http://bio-bwa.sourceforge.net/bwa.shtml.
- CIRCexplorer2 Zhang XO, Dong R, Zhang Y, Zhang JL, Luo Z, Zhang J, Chen LL, Yang L. (2016). Diverse alternative back-splicing and alternative splicing landscape of circular RNAs. Genome Res. 2016 Sep;26(9):1277-87. Available at: https://doi.org/10.1101/gr.202895.115. Download: https://circexplorer2.readthedocs.io/en/latest/tutorial/setup/
- circRNA finder Westholm, J.O., Lai, E.C., et al. (2016). Genome-wide Analysis of Drosophila Circular RNAs Reveals Their Structural and Sequence Properties and Age-Dependent Neural Accumulation Westholm et al. Cell Reports. Available at: https://doi.org/10.1016/j.celrep.2014.10.062. Download: https://github.com/orzechoj/circRNA_finder
- CIRIquant Zhang, J., Chen, S., Yang, J. et al. (2020). Accurate quantification of circular RNAs identifies extensive circular isoform switching events. Nat Commun 11, 90. Available at: https://doi.org/10.1038/s41467-019-13840-9. Download: https://github.com/bioinfo-biols/CIRIquant
- DCC Jun Cheng, Franziska Metge, Christoph Dieterich, (2016). Specific identification and quantification of circular RNAs from sequencing data, Bioinformatics, 32(7), 1094–1096. Available at: https://doi.org/10.1093/bioinformatics/btv656. Download: https://github.com/dieterich-lab/DCC
- find circ Memczak, S., Jens, M., Elefsinioti, A., Torti, F., Krueger, J., Rybak, A., Maier, L., Mackowiak, S. D., Gregersen, L. H., Munschauer, M., Loewer, A., Ziebold, U., Landthaler, M., Kocks, C., le Noble, F., & Rajewsky, N. (2013). Circular RNAs are a large class of animal RNAs with regulatory potency. Nature, 495(7441), 333–338. Available at: https://doi.org/10.1038/nature11928. Download: https://github.com/marvin-jens/find_circ
- HISAT2 Kim, D., Paggi, J.M., Park, C. et al. (2019). Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol 37, 907–915 (2019). Available at: https://doi.org/10.1038/s41587-019-0201-4. Download: http://daehwankimlab.github.io/hisat2/download/
- MapSplice2 Wang, K., Liu J., et al. (2010) MapSplice: Accurate mapping of RNA-seq reads for splice junction discovery, Nucleic Acids Research, 38(18), 178. Avaialable at: https://doi.org/10.1093/nar/gkq622. Download: http://www.netlab.uky.edu/p/bioinfo/MapSplice2Download
- miRanda Enright, A.J., John, B., Gaul, U. et al. (2003). MicroRNA targets in Drosophila. Genome Biol 5, R1. Available at: https://doi.org/10.1186/gb-2003-5-1-r1. Download: http://cbio.mskcc.org/miRNA2003/miranda.html.
- Picard Broad Institute (Unpublished). Download: http://broadinstitute.github.io/picard/
- R: R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Download: https://www.R-project.org/.
- biomaRt Durinck S, Spellman PT, Birney E, Huber W. (2009). Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat Protoc. 4(8):1184-91. Available at: https://doi.org/10.1038/nprot.2009.97. Download: https://bioconductor.org/packages/release/bioc/html/biomaRt.html
- circlize Zuguang Gu, Lei Gu, Roland Eils, Matthias Schlesner, Benedikt Brors (2014). circlize implements and enhances circular visualization in R , Bioinformatics, 30,(19) 2811–2812. Available at: https://doi.org/10.1093/bioinformatics/btu393. Download: https://cran.r-project.org/web/packages/circlize/index.html
- DESeq2 Love, M.I., Huber, W. & Anders, S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. Available at: https://doi.org/10.1186/s13059-014-0550-8. Download: https://bioconductor.org/packages/release/bioc/html/DESeq2.html
- EnhancedVolcano Blighe K, Rana S, Lewis M (2020). EnhancedVolcano: Publication-ready volcano plots with enhanced colouring and labeling. Download: https://bioconductor.org/packages/release/bioc/html/EnhancedVolcano.html
- ggplot2 Wickham H (2016). ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York. ISBN 978-3-319-24277-4, Download: https://ggplot2.tidyverse.org.
- ggpubr Kassambara A. (2020). ggpubr: 'ggplot2' Based Publication Ready Plots. Download: https://rpkgs.datanovia.com/ggpubr/
- ihw Ignatiadis, N., Klaus, B., Zaugg, J. et al. (2016). Data-driven hypothesis weighting increases detection power in genome-scale multiple testing. Nat Methods 13, 577–580. Available at: https://doi.org/10.1038/nmeth.3885. Download: https://bioconductor.org/packages/release/bioc/html/IHW.html
- PCAtools Blighe K, Lun A (2020). PCAtools: PCAtools: Everything Principal Components Analysis. Download: https://bioconductor.org/packages/release/bioc/html/PCAtools.html
- pheatmap Kolde, R. (2019) Pretty Heatmaps. Download: https://cran.r-project.org/package=pheatmap
- pvclust Suzuki R., Shimodaira H., (2006). Pvclust: an R package for assessing the uncertainty in hierarchical clustering, Bioinformatics, 22(12), 1540–1542. Available at: https://doi.org/10.1093/bioinformatics/btl117. Download: https://cran.r-project.org/web/packages/pvclust/index.html
- tximport Soneson C, Love MI, Robinson MD (2015). Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Research, 4. Avaialable at: https://doi.org/10.12688/f1000research.7563.1. Download: http://bioconductor.org/packages/release/bioc/html/tximport.html
- SAMtools Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., … 1000 Genome Project Data Processing Subgroup. (2009). The Sequence Alignment/Map format and SAMtools. Bioinformatics , 25(16), 2078–2079. https://doi.org/10.1093/bioinformatics/btp352. Download: http://www.htslib.org/
- Segemehl Hoffmann S, Otto C, Kurtz S, Sharma CM, Khaitovich P, Vogel J, Stadler PF, Hackermueller J: "Fast mapping of short sequences with mismatches, insertions and deletions using index structures", PLoS Comput Biol (2009) vol. 5 (9) pp. e1000502. Available at: https://doi.org/10.1371/journal.pcbi.1000502. Download: https://www.bioinf.uni-leipzig.de/Software/segemehl/
- STAR Dobin, A., Davis, C. A., Schlesinger, F., Drenkow, J., Zaleski, C., Jha, S., Batut, P., Chaisson, M., & Gingeras, T. R. (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics (Oxford, England), 29(1), 15–21. Available at: https://doi.org/10.1093/bioinformatics/bts635. Download: https://github.com/alexdobin/STAR
- StringTie Pertea, M., Pertea, G., Antonescu, C. et al. (2015). StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol 33, 290–295. Available at: https://doi.org/10.1038/nbt.3122. Download: https://ccb.jhu.edu/software/stringtie/
- TargetScan Agarwal V, Bell GW, Nam JW, Bartel DP. (2015). Predicting effective microRNA target sites in mammalian mRNAs. Elife, 4:e05005. Available at: https://doi.org/10.7554/elife.05005. Download: http://www.targetscan.org/cgi-bin/targetscan/data_download.vert72.cgi
- ViennaRNA Lorenz, R., Bernhart, S.H., Höner zu Siederdissen, C. et al. (2011). ViennaRNA Package 2.0. Algorithms Mol Biol 6, 26. Available at: https://doi.org/10.1186/1748-7188-6-26. Download: https://www.tbi.univie.ac.at/RNA/#download
Dong Cao (2021). An autoregulation loop in fust-1 for circular RNA regulation in Caenorhabditis elegans. Biorxiv. Available at: https://doi.org/10.1101/2021.03.22.436400.