bigSCale is a complete framework for the analysis and visualization of single cell data. It allows to cluster, phenotype, perform pseudotime analysis, infer gene regulatory networks and reduce large datasets in smaller datasets with higher quality.
Why using bigSCale 2?
- bigSCale2 features the most sensitive and accurate marker detection and classification. No method is used to reduce dimensions, every bit of information is retained.
- bigSCale2 allows to infer the gene regulatory networks for any single cell dataset.
- bigSCale2 allows to compress large datasets of any size into a smaller datasets of higher quality, without loss of information. One millions cells are too many to be analyzed by your favourite tool ? Reduce them to a dataset with less cells of increased quality and go for it!
Quick Start
bigSCale is formed by three sub-tools which can be used either independently or in synergy. Each sub-tool has its own tutorial.
bigSCale 2 Core allows to cluster, phenotype and perform pseudo-time analysis. It's the main tool of bigSCale, published in Iacono 2018.
bigSCale 2 GRN is the newest addition: it is the module to infer gene regulatory networks from single cell data. Iacono 2019
bigSCale 2 iCells allows to reduce the dimension of any given large dataset (also millions of cells, wothout any loss of information) so that it can be easily and quickly analyzed by any tool.The resulting dataset has less cells with higher quality, so it can be analyzed better. It DOES NOT require any external tool such as the loom framework.
- Quick start and basic use
- Running bigSCale
- Visualizing results
- Advanced use
- Item 2a
- Link to Header
READ BEFORE STARTING bigSCale2 is a special tool designed to have maximum accuracy in clustering and detection of markers. bigSCale2 achieves extreme accuracy also because it does not use any dimensionality reduction. If you have a large number of cells and you want to cluster/phenotype it with bigSCale2 Core, then first process it with the tool bigSCale2 iCells. As a rule of thumb, you can analyze directly (without process with iCells) up to 20K cells if you have 16 Gb of RAM or up to 40K cells if you have 32b of RAM.
bigSCale2 works with the SingleCellExperiment class. This class is a container meant to store in an organized way single cell data.
bigScale2 requires two elements to be present in the single cell class: the counts counts() and the gene names rownames().
The counts must be raw counts! The genes must no be filtered, aside from removing, if you want, the gene with all zero values.
Let us first load an example dataset : 3005 single cells from adult mouse brain Zeisel 2015
data(sce)
As you can see, the sce object contains the expression values for 19972 genes in 3005 cells. In its most basic use, bigScale is run with just one command sce=bigscale(sce) which will automatically perform all the analysis. However, for time reasons, we will instruct bigSCale2 to perform a quick analysis to save us time, by specifying speed.preset='fast', which greatly reduces the the time required to compute markers and differentially expressed genes, but at the expenses of the quality and accuracy (uses only wilcoxon test). In a real analysis we reccomand not to use this setting, and achieve maximum accuracy leaving as default speed.preset='slow' (leaving speed.preset='fast' works well when you have lots of cells, say>15K or 20K).
sce=bigscale(sce,speed.preset='fast')
The analysis are now all complete and stored again in the sce object. In the next part we'll see how to visualize the results.
bigSCale2 feature a basic set of plot types to visualize the main results of clustering and phenotyping.
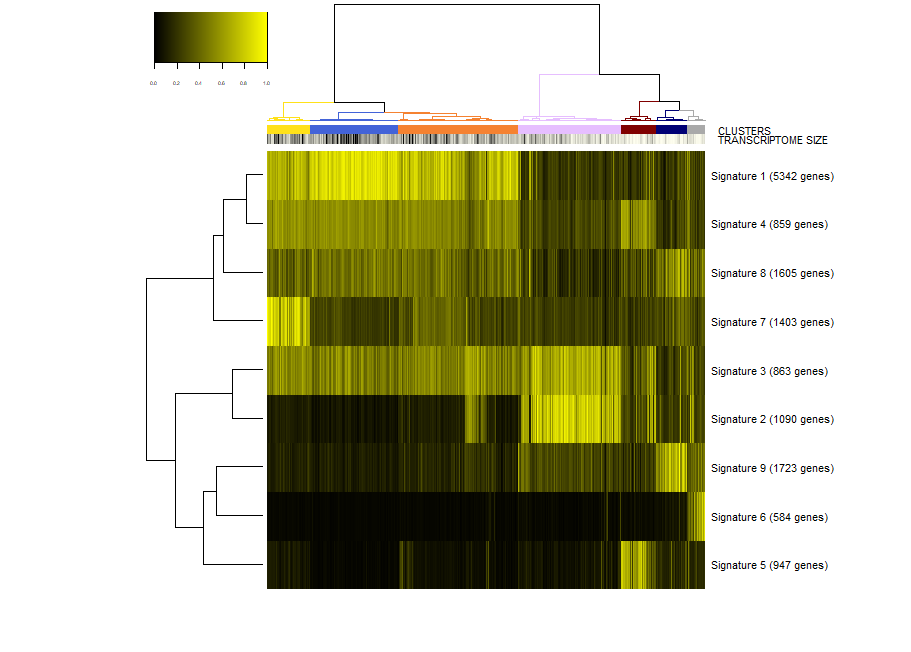
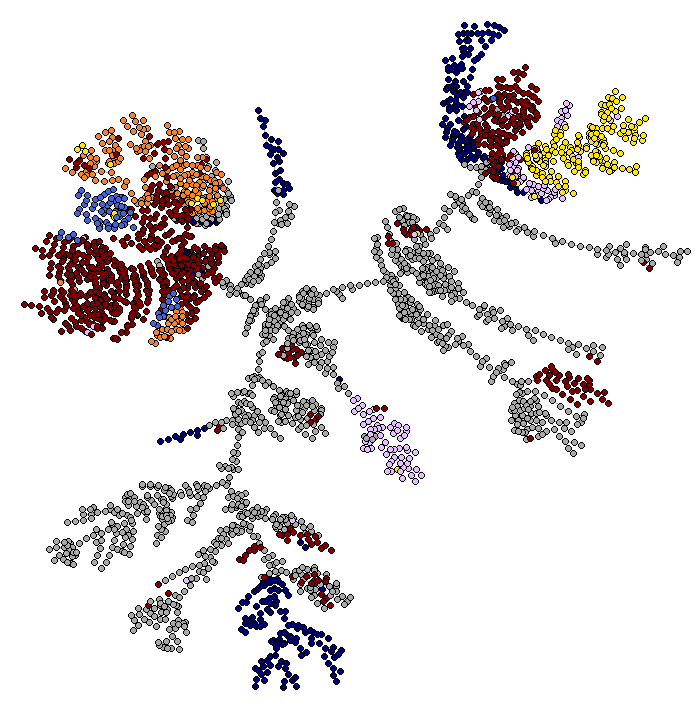
First, we make a plot of the clusters and signatures of coexpressed genes.
viewSignatures(sce)
In this plot you can see
- The dendrogram representing how the cells are phenotypically organized and clustered
- Colored bars representing the clusters, the library size (meant as a proxy to transcriptome size/complexity) and the pseudotime of the cells. An additional color bar is displayed for any user custom
colData()(for example, sample batches, conditions and so on ...). For custom usercolData, the color codes are automatically chosen upoen the type of data (numeric or factor). - The clustered signatures of coexpressed genes alogside their size. Here, all the genes differentially expressed are organized in signatures of co-expressed genes.
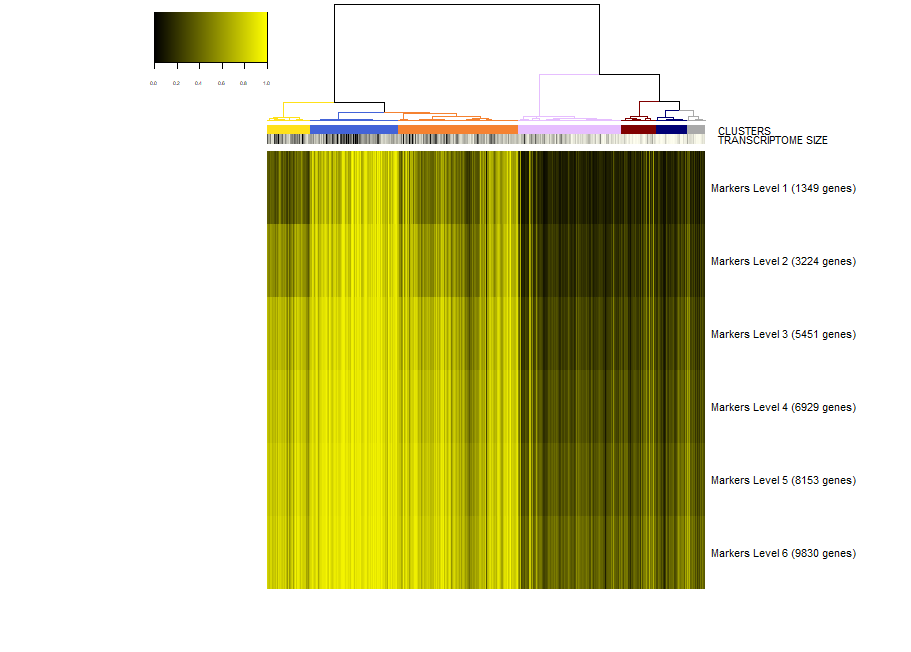
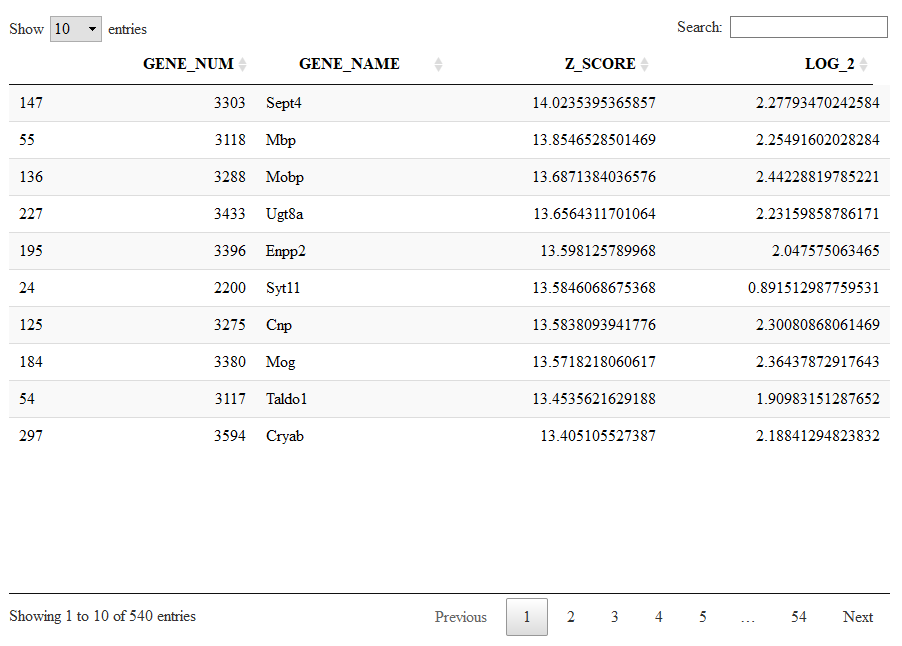
Next, we would like to inspect the markers of a specific cluster, let's say cluster 2. To this end, we run.
viewSignatures(sce,selected.cluster=2)
Now, the plot is the same as before, but in place of the signatures of coexpressed genes we see the markers of cluster 2 stratified by level of specificity. If you read my paper Iacono 2018 then you'll know what this means. Shortly, markers of level 1 are the most specific to a given cluster. Level 1 means that this 417 genes are expressed only in cluster 2 . However, shared markers are also very important in biology. Think to all the markers shared by neuronal cell types as opposed to glial cell types. Shared genes are represented in biSCale by markers of increasing levels. Markers of level 2 (629 genes) are markers shared between cluster 2 and at most another cluster. Markers of level 3 are shared shared between cluster 2 and at most two other clusters, and so on. These markers of higher levels are typically lost by other computational tools.
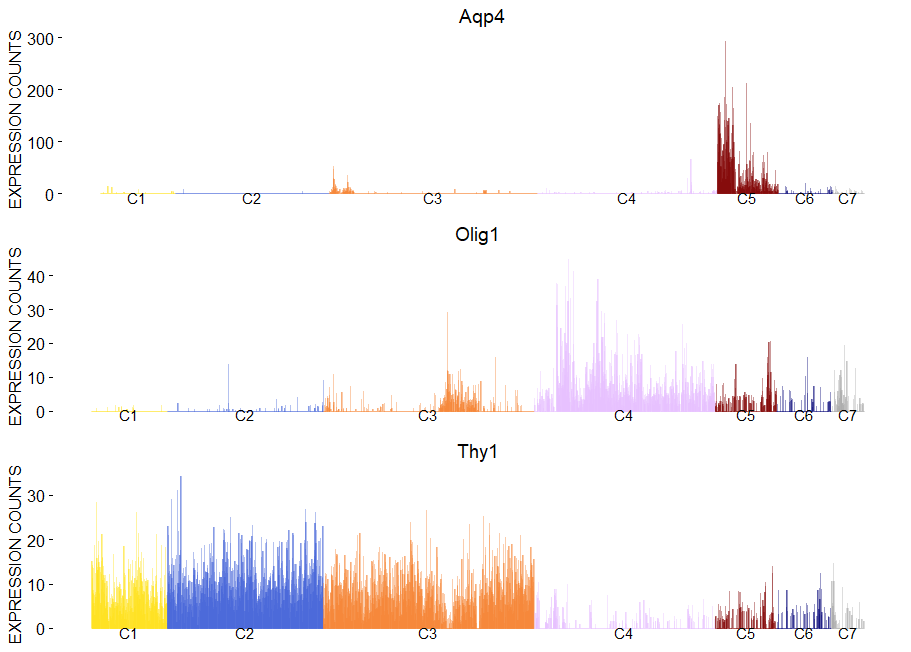
To plot gene expression at single cell level with colored clusters. This plot works well in synergy with the plot of the hierachical clustering.
viewGeneBarPlot(sce,gene.list = c('Aqp4','Olig1','Thy1'))
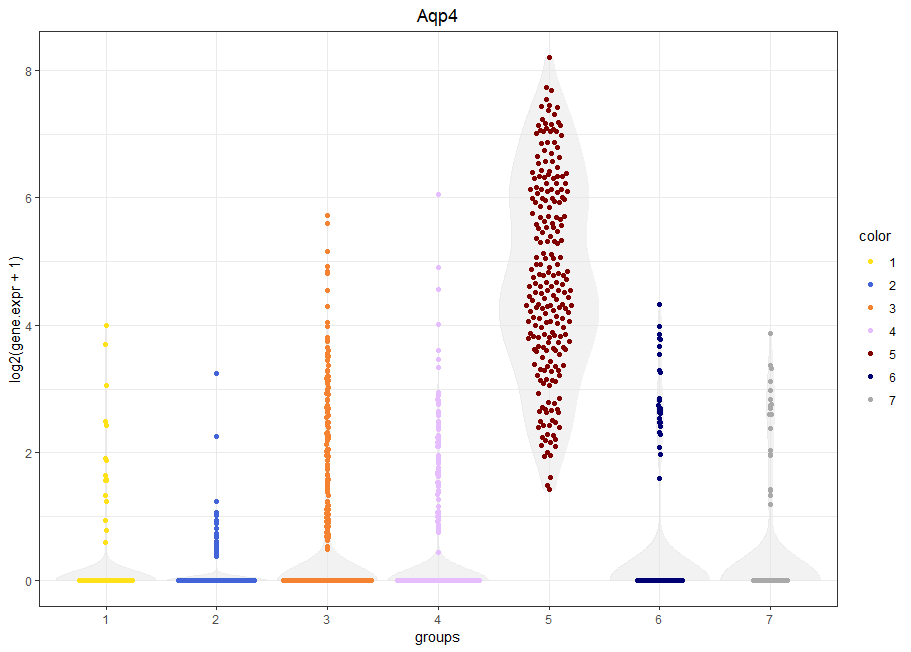
viewGeneViolin(sce,'Aqp4')
To have a look to the markers found by bigscale we retrive Mlist from the single cell object. Mlist is a 2 dimensional list containing for each cluster the markers of the different levels. Let's inspect the markers of level 1 (most specific) of cluster 4 (oligodendrocytes). We will take advantage of the package DT for interactive visualization.
Running the next command line we will see the markers specific to cluster 4 sorted from the highest (most significant) to the lowest (less significant) Zscore.
Mlist=getMarkers(sce)
DT::datatable(Mlist[[4,1]])
Let us now check other genes marking oligodendrocytes (cluster 5) which are shared with other clusters. We check markers of level 5 to include genes shared with at most 4 other clusters.
DT::datatable(Mlist[[4,5]])
Here we can browse for Cd9, which has now a significant Z-score. Cd9 is a surface receptor expressed in oligodendrocytes but also in all the other glial cells.
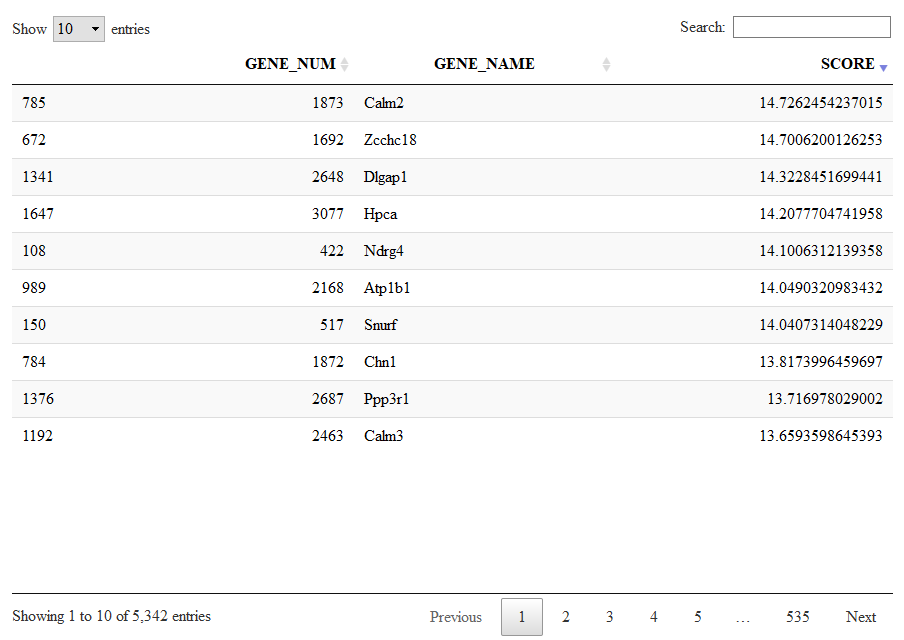
Alternatevely to a cluster-based organization of the markers (as shown before, we have clusters and we have levels) there is also a more compact organization of markers into lists of co-expressed genes. This are the same lists shown with viewSignatures(sce).
Let us give a look to signature 1, a large list of 5432 genes expressed in neurons.
Signatures=getSignatures(sce)
DT::datatable(Signatures[[1]])
Keep in mind that we calculated these markers and signatures with the speed.preset='fast' which means low quality. This plots nd tables are for demonstration purposes only.
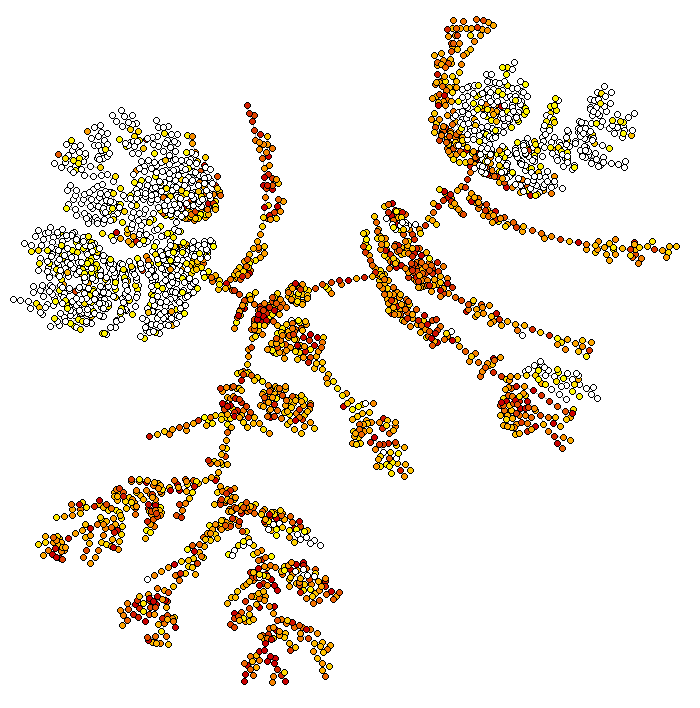
bigSCale2 can infer pseudotime and plot differentiation trajectories. By default, the flag pseudotime=TRUE tells bigSCale to compute psudotime.
It does not make much biological sense to compute pseudtime of fully differentiated brain cells but this is just a technical demonstration. To view the results of the pseudotime we run:
ViewPseudo(sce,color.by = 'pseudo') # colors by pseudotime
ViewPseudo(sce,color.by = 'clusters') # colors by clusters
ViewPseudo(sce,color.by = 'Stmn3') # colors by a specific gene
The bigscale() function which performs all the analysis at once is actually a wrapper for a series of sub-functions.
Here we see how to run each part of the analysis separatly, to customize the pipeline.
We start again from the same dataset of the basic tutorial.
data(sce)
Now, the first step is to pre-process the dataset. This step removes null genes and creates a number of internal variables needed by bigSCale2. In the future I will add the possibility here to specify a custom library size.
sce=preProcess(sce)
Next three passages are non customizable (at least for the moment), we run them at once:
sce = storeNormalized(sce) # stores normalized data
sce=setModel(sce) # computes the numerical model
sce = storeTransformed(sce) # stores transformed expression data (needed for some plots)
A very important thing is to check that the numerical model of bigSCale2 is not completley messed up. In my experience it is a rare case but can happen. To check the computed model we run:
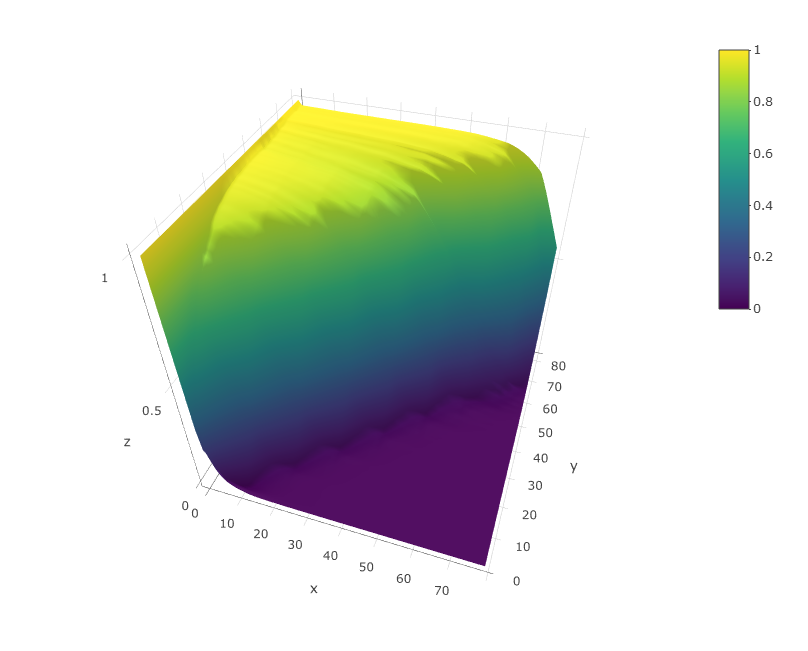
viewModel(sce)
The model should look similar to the upper figure: a kind of sigmoidal surface decreasing more or less steeply in the diagonal. The sharper the decrese of the surface along the diagonal, the better the quality of the dataset. If the plot is very different from this one contact me. I will probably add in the close future an automated quality check of the model.
Now we compute the highly variable genes and we calculate cell to cell distances.
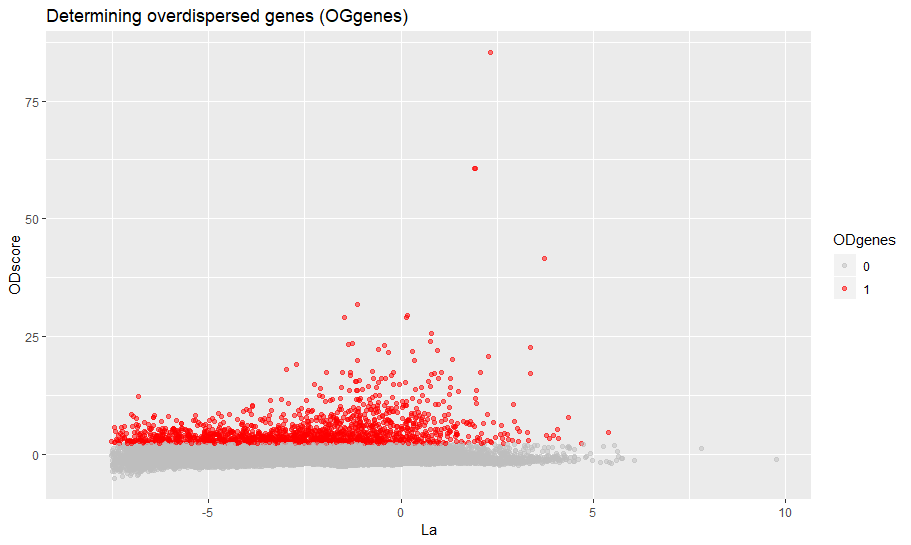
min_ODscore is the Z-score treshold for selecting the genes (default 2.33). Increase it to be more stringent (less genes) and viceversa. It is also possible to exclude specific intervals of expression from highly variable genes (parameter use.expm check documentation of setODgenes). For example, exclude highly expressed genes (e.g. Ribosomal, Mitochondrial) from being selected.
sce = setODgenes(sce,min_ODscore=2.33)
You can visually inspect the selected highly variable genes with:
viewODgenes(sce)
Next we compute the distances, nothing to customize here.
sce=setDistances(sce)
We compute and store the TSNE data, if you want to see TSNE plots of course. Otherwise skip.
sce=storeTsne(sce)
Now we cluster the data. Here you can make a couple of customizations to vary the number of clusters or even providing your own clusters. Check the help of the setClusters to see how to do.
sce=setClusters(sce)
If you want to make also pseuotime analysis then run:
sce=storePseudo(sce)
With the next command we compute the differential expression for all genes. This is generally the slowest part of the analysis. You can trade speed versus accuracy by changing the parameter speed.preset. I reccomand speed.preset='slow', which is maximum accuracy with long computational time. I already discussed this parameter in the basic introduction.
sce=computeMarkers(sce,speed.preset='slow')
Now we organize all the genes into markers and signatures of co-expression. cutoff is a Z-score which filters the genes and retains nly thosw with significant changes of expression. Inrease it if you want to be more stringent or viceversa.
sce=setMarkers(sce,cutoff=3)
Finally, we restore some matrices from the virtual memory and we have competed our analysis.
sce=restoreData(sce)
Important: This short tutorial will show how to infer regulatory networks from single cell data and quantify gene centrality (and some other stuff). Clearly, graph theory is a large word, and there are lots of analysis other than node centrality that you can potentially perform on the networks. We leave this up to you!
bigSCale2 allows to infer the putative gene regulatory network (GRN) from any single cell dataset. Here, we will show how to infer the GRNs from healthy pancreas and from type 2 diabetes (T2D) pancreas and compare them. This is the same pancreas dataset used in our paper, from Segerstolpe 2016. Let's load the data.
data("pancreas")
Now we have in our sesssion two matrices with the expression counts for healthy (1313) and T2D (1178) cells, expr.ctl and expr.t2d, and the gene names gene.names. This is all we need to infer the networks. Please note that for the moment bigSCale2 supports only Gene Symbols or Ensembl, Mouse and Human. Contact me if you want that I add other species or nomenclatures.
There is only one parameter that you will change when doing a basic use of our tool. The parameter is clustering and it works as tradoff between accuracy and time. To put it simply:
clustering='recursive' Is the default. Maximum accuracy, maximum time. If you are okay with leaving overnight then run it.
clustering='direct' Good accuracy, mimimum time. The fastest way to have results, if you are in a hurry.
As in the tutorial we are in a hurry we will do:
results.ctl=compute.network(expr.data = expr.ctl,gene.names = gene.names,clustering = 'direct')
results.t2d=compute.network(expr.data = expr.t2d,gene.names = gene.names,clustering = 'direct')
The output of compute.network() is a list with a series of useful elements. I will not discuss here all the elements, I added a clear explanation about them in the help of the function compute.network().
Let's have a look to the networks.
results.ctl$graph
IGRAPH f4962c7 UN-- 8887 89936 --
+ attr: name (v/c)
+ edges from f4962c7 (vertex names):
[1] CLIC4 --RARB CLIC4 --ABLIM3 CLIC4 --QKI
[4] CLIC4 --MDFI CLIC4 --EPHX2 CLIC4 --MSL3
[7] CLIC4 --HNRNPF CLIC4 --FOSL1 CLIC4 --CARD16
[10] CLIC4 --ELK3 CLIC4 --THBS1 CLIC4 --YWHAB
[13] AGBL4 --PEG3 NECAP2--LMNA NECAP2--SF3B4
[16] NECAP2--ARID5A NECAP2--BAK1 NECAP2--ACTB
[19] NECAP2--TBRG4 NECAP2--ILK NECAP2--RFXANK
[22] NECAP2--MAFF TGFBR3--SIX2 TGFBR3--KIAA1211
+ ... omitted several edges
results.t2d$graph
IGRAPH ad2e341 UN-- 9407 97496 --
+ attr: name (v/c)
+ edges from ad2e341 (vertex names):
[1] CLIC4--IFI16 CLIC4--CYR61 CLIC4--PHGDH CLIC4--CELF3
[5] CLIC4--FHL2 CLIC4--ACTR3 CLIC4--CSRNP3 CLIC4--FN1
[9] CLIC4--SP100 CLIC4--EIF4E2 CLIC4--CYP1B1 CLIC4--INSIG2
[13] CLIC4--RAF1 CLIC4--CTNNB1 CLIC4--FSTL1 CLIC4--REST
[17] CLIC4--ENC1 CLIC4--DIMT1 CLIC4--NFYA CLIC4--SYNCRIP
[21] CLIC4--RFX6 CLIC4--CHCHD3 CLIC4--CBX3 CLIC4--CAV1
[25] CLIC4--MTDH CLIC4--CLU CLIC4--MSL3 CLIC4--MSN
[29] CLIC4--HMGN5 CLIC4--BEX1 CLIC4--MOSPD1 CLIC4--LMO1
+ ... omitted several edges
We have succesfully generated the networks. Control network has 8887 nodes (genes) and 89936 edges. T2D network has 9407 nodes and 97496 edges. Please remember that we generated these networks using the fastest option. When dealing with datasets so small it would be much better to use clustering='recursive'.
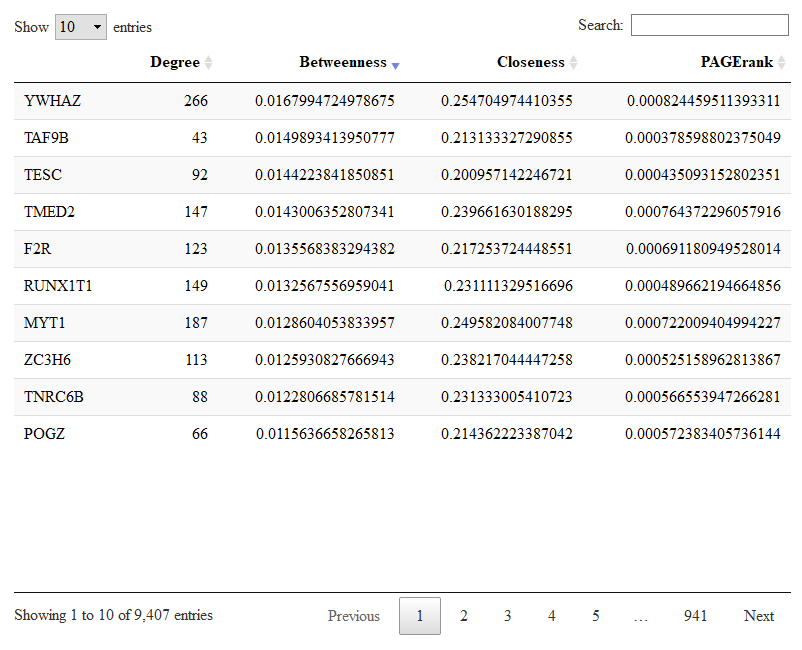
bigSCale2 has already calculated the gene (node) centralities and stored them in the output list. Centralities are a measure of the importance of a gene in the regulatory netowork. You should read our paper to know more. Let us give a look to the most central genes in the T2D network.
DT::datatable(results.t2d$centrality)
Here you have the table with the four centralities for each gene. You can sort each centrality to have a look to the most central genes. In the picture we sorted the betweenness. Genes with high betweenness are important imformation bottlenecks.
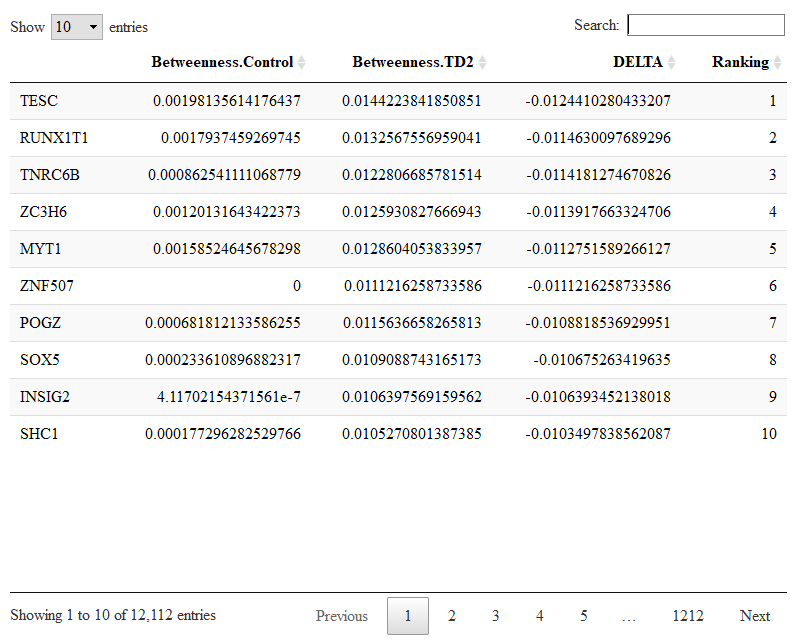
To compare changes in node centralities from control to T2D networks we run.
comparison=compare.centrality(list(results.ctl$centrality,results.t2d$centrality),c('Control','TD2'))
The input of compare.centrality() is a list whose elements are the previously calculated centralities. We also provide the complete names of the conditions which will be used to annotate the output. The output of compare.centrality() is a list of data.frames. Each data.frame stores the genes ranked for their change in one given centrality (degree, betweenness, closeness, pagerank in order).
Let us check the genes with changes in betweenness.
DT::datatable(comparison$betweenness)
In our paper we show how to integrate node cenrality with differential expression (DE). The idea is simple. The genes with the highest centrality in the regulatory network are the most biologically important. Exploiting the known centrality, you can extract the DE genes with maximal biological relevance.
Let's say that you are particularly interested in a specific gene. Aside from computing its centralities, you can also have a closer look to its correlations using the $correlations matrix in the output of compute.network() (check the help of the function). In this way you can check the correlations between your gene of interest and the rest of the trancriptome. If you have more than one network, you can compare how these correlations change.
I am not providing custom visualization tools. The networks are in igraph format, so you can use the igraph visualization tools.
Networks are automatically generated by retaining only the most significant correlations. By default, the parameter quantile.p=0.998 retains only the top 0.2% correlations. These selected correlations will become the edges of the raw network. We use a percentile selection instead of a fixed value selection (for example, correlations > 0.8 become edges) for reasons explained in our paper.
Most of the times, selecting the top 0.2% correlations works fine. However, it could be that in some cases the overall distribution of correlations is pretty poor (meaning good correlations are rare) and that the top 0.2% includes correlations which are not good. When the top 0.2% percentile corresponds to a cutoff in the correlations which is lower than 0.7 the tool displays a warning and suggests to increase quantile.p or change some other settings.
Let's say we want to be more strict with the selection of the edges, we want to use 0.999 instead of 0.998 for quantile.p for the control network.
results.ctl=compute.network(previous.output = results.ctl,quantile.p = 0.999)
Also, if you believe that your network is too dense (too many edges) or not dense enough (not enough edges), you can re-run the analysis increase or decreasing quantile.p, respectevely.