Although recent efforts have shown that structural variations (SVs) can disrupt the 3D genome organization and induce enhancer-hijacking, no computational tools exist to detect such events from chromatin interaction data, such as Hi-C. Here, we develop NeoLoopFinder, a computational framework to identify the chromatin interactions induced by SVs, such as inter-chromosomal translocations, large deletions, and inversions. Our framework can automatically reconstruct local Hi-C maps surrounding the breakpoints, normalize copy number variation and allele effects, and capture local optimal signals. We applied NeoLoopFinder in Hi-C data from 50 cancer cell lines and primary tumors and identified tens of recurrent genes associated with enhancer-hijacking in different cancer types. To validate the algorithm, we deleted hijacked enhancers by CRISPR/Cas9 and showed that the deletions resulted in the reduction of the target oncogene expression. In summary, NeoLoopFinder is a novel tool for identifying potential tumorigenic mechanisms and suggesting new diagnostic and therapeutic targets.
Wang, X., Xu, J., Zhang, B., Hou, Y., Song, F., Lyu, H., Yue, F. Genome-wide detection of enhancer-hijacking events from chromatin interaction data in re-arranged genomes. Nat Methods. 2021.
NeoLoopFinder and all the dependencies can be installed using conda or pip:
$ conda config --add channels defaults $ conda config --add channels bioconda $ conda config --add channels conda-forge $ conda create -n neoloop python=3.7.1 cython=0.29.13 cooler=0.8.6 numpy=1.17.2 scipy=1.3.1 joblib=0.13.2 scikit-learn=0.20.2 networkx=1.11 pyensembl=1.8.0 matplotlib=3.1.1 pybigwig=0.3.17 pomegranate=0.10.0 $ conda activate neoloop $ conda install -c r r=3.5.1 rpy2=2.9.4 r-mgcv=1.8_23 $ pip install neoloop TADLib==0.4.2 coolbox==0.1.7
neoloop-finder is distributed with 8 scripts. You can learn the basic usage of each script
by typing command [-h] in a terminal window, where "command" is one of the following
script names:
calculate-cnv
Calculate the copy number variation profile from Hi-C map using a generalized additive model with the Poisson link function
segment-cnv
Perform HMM segmentation on a pre-calculated copy number variation profile.
correct-cnv
Remove copy number variation effects from cancer Hi-C.
simulate-cnv
Simulate CNV effects on a normal Hi-C. The inputs are the Hi-C matrix of a normal cell in .cool format, the Hi-C matrix of a cancer cell in .cool format, and the CNV segmentation file of the same cancer cell in bedGraph format.
assemble-complexSVs
Assemble complex SVs. The inputs are a list of simple SVs and the Hi-C matrix of the same sample.
neoloop-caller
Identify neo-loops across SV breakpoints. The required inputs are the output SV assemblies from
assemble-complexSVsand the corresponding Hi-C map in .cool format.neotad-caller
Identify neo-TADs. The inputs are the same as
neoloop-caller.searchSVbyGene
Search SV assemblies by gene name.
As copy number variations (CNVs) can distort Hi-C signals in cancer cells, we proposed a modified
matrix balancing algorithm to remove such effects along with other systematic biases including mappability,
GC content, and restriction fragment sizes. In our implementation, you can perform this CNV normalization by
sequentially running calculate-cnv, segment-cnv, and correct-cnv. The Hi-C map in
.cool format is the only required input to this pipeline, and the
bias vector returned by this algorithm will be stored in the "sweight" column in the bins
table of the cool file.
By default, assemble-complexSVs, neoloop-caller, and neotad-caller will use the "sweight" column to
normalize the Hi-C matrix. However, you can change this option to ICE normalization by specifying --balance-type ICE.
The input SV file to the command assemble-complexSVs should contain following 6 columns separated by tab:
chr7 chr14 ++ 14000000 37500000 translocation chr7 chr14 -- 7901149 37573191 translocation
- chrA: The chromosome name of the 1st breakpoint.
- chrB: The chromosome name of the 2nd breakpoint.
- orientation: The orientation type of the fusion, one of ++, +-, -+, or --.
- b1: The position of the 1st breakpoint on chrA.
- b2: The position of the 2nd breakpoint on chrB.
- type: SV type. Allowable choices are: deletion, inversion, duplication, and translocation.
This tutorial will cover the basic usage of assemble-complexSVs, neoloop-caller and the
visualization module.
First, change your current working directory to the test folder and download the Hi-C contact map in K562:
$ cd test $ wget -O K562-MboI-allReps-hg38.10K.cool https://www.dropbox.com/s/z3z5bye1tuywf18/K562-MboI-allReps-hg38.10K.cool?dl=0
To detect and assemble complex SVs in K562, submit the command below:
$ assemble-complexSVs -O K562 -B K562-test-SVs.txt -H K562-MboI-allReps-hg38.10K.cool
The job should be finished within 1 minute, and all candidate local assemblies will be reported into a TXT file named "K562.assemblies.txt":
A0 translocation,22,23290555,+,9,130731760,- translocation,9,131280137,+,13,108009063,+ deletion,13,107848624,-,13,93371155,+ 22,22300000 13,93200000 A1 translocation,9,131280000,+,13,93252000,- deletion,13,93371155,+,13,107848624,- 9,130720000 13,108030000 A2 translocation,22,23290555,+,9,130731760,- translocation,9,131280000,+,13,93252000,- 22,22300000 13,93480000 A3 translocation,22,23290555,+,9,130731760,- translocation,9,131199197,+,22,16819349,+ 22,22300000 22,16240000 C0 deletion,13,93371155,+,13,107848624,- 13,93200000 13,108030000 C1 translocation,22,16819349,+,9,131199197,+ 22,16240000 9,130710000 C2 translocation,22,23290555,+,9,130731760,- 22,22300000 9,131290000 C3 translocation,9,131280000,+,13,93252000,- 9,130720000 13,93480000 C4 translocation,9,131280137,+,13,108009063,+ 9,130720000 13,107810000
Then you can detect neo-loops on each assembly using the neoloop-caller command:
$ neoloop-caller -O K562.neo-loops.txt -H K562-MboI-allReps-hg38.10K.cool --assembly K562.assemblies.txt --no-clustering --prob 0.95
Wait ~1 minute... The loop coordinates in both shuffled (neo-loops) and undisrupted regions near SV breakpoints will be reported into "K562.neo-loops.txt" in BEDPE format:
$ head K562.neo-loops.txt chr13 93270000 93280000 chr13 107860000 107870000 A0,130000,1 chr13 93270000 93280000 chr13 107870000 107880000 A0,140000,1 chr13 93270000 93280000 chr13 107980000 107990000 A0,250000,1 chr13 93280000 93290000 chr13 107860000 107870000 A0,120000,1 chr13 93280000 93290000 chr13 107870000 107880000 A0,130000,1,C0,130000,1 chr13 93280000 93290000 chr13 107880000 107890000 A0,140000,1 chr13 93280000 93290000 chr13 107970000 107980000 A0,230000,1 chr13 93290000 93300000 chr13 107860000 107870000 A1,110000,1,C0,110000,1 chr13 93290000 93300000 chr13 107870000 107880000 A1,120000,1,A0,120000,1,C0,120000,1 chr13 93300000 93310000 chr13 107870000 107880000 C0,110000,1
The last column records the assembly IDs, the genomic distance between two loop anchors on the assembly and whether this is a neo-loop. For example, for the 1st row above, the loop was detected on the assemblies "A0", the genomic distance between the two anchors on this assembly is 130K (note that the distance on the reference genome is >14Mb), and it is a neo-loop as indicated by "1".
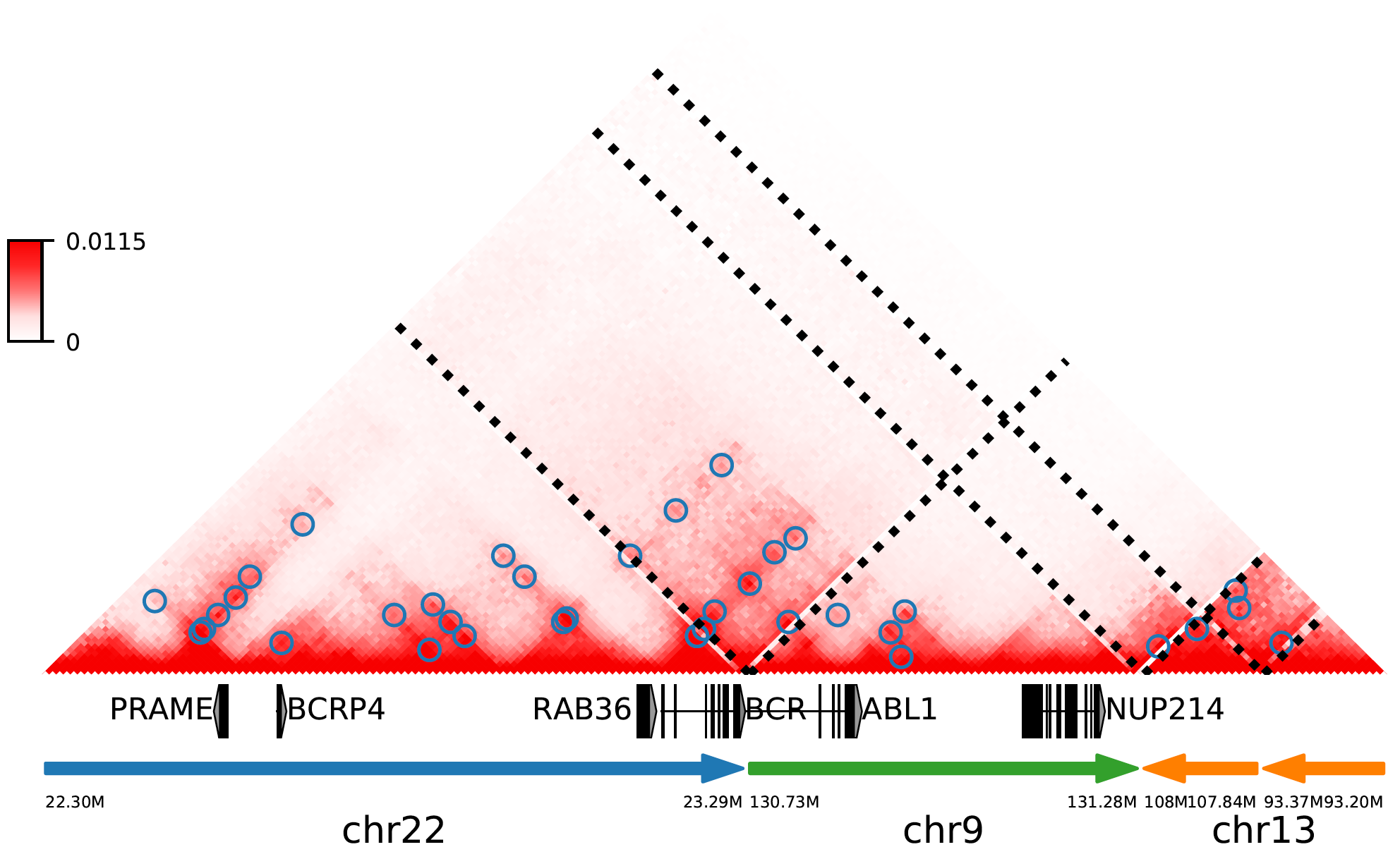
Finally, let's reproduce the figure 1b using the python code below (we recommend using ipython to explore it interactively):
In [1]: from neoloop.visualize.core import *
In [2]: import cooler
In [3]: clr = cooler.Cooler('K562-MboI-allReps-hg38.10K.cool')
In [4]: assembly = 'A0 translocation,22,23290555,+,9,130731760,- translocation,9,131280137,+,13,108009063,+ deletion,13,107848624,-,13,93371155,+ 22,22300000 13,93200000'
In [5]: vis = Triangle(clr, assembly, n_rows=3, figsize=(7, 4.2), track_partition=[5, 0.4, 0.5], correct='sweight')
In [6]: vis.matrix_plot(vmin=0)
In [7]: vis.plot_chromosome_bounds(linewidth=2.5)
In [8]: vis.plot_loops('K562.neo-loops.txt', face_color='none', marker_size=40, cluster=True)
In [9]: vis.plot_genes(filter_=['PRAME','BCRP4', 'RAB36', 'BCR', 'ABL1', 'NUP214'],label_aligns={'PRAME':'right','RAB36':'right'}, fontsize=9)
In [10]: vis.plot_chromosome_bar(name_size=11, coord_size=4.8)
In [11]: vis.outfig('K562.A0.pdf')