tidysdm is a package to make it easy to fit Species Distribution
Models in a tidymodels framework.
You can install the development version of tidysdm from GitHub with:
# install.packages("devtools")
devtools::install_github("rdinnager/tidysdm")library(tidysdm)
#> Loading required package: parsnip
#> Loading required package: recipes
#> Loading required package: dplyr
#>
#> Attaching package: 'dplyr'
#> The following objects are masked from 'package:stats':
#>
#> filter, lag
#> The following objects are masked from 'package:base':
#>
#> intersect, setdiff, setequal, union
#>
#> Attaching package: 'recipes'
#> The following object is masked from 'package:stats':
#>
#> step
set.seed(112233)We will use data from the ENMTools package to demonstrate how
tidysdm works.
library(ENMTools)
#> Loading required package: raster
#> Loading required package: sp
#>
#> Attaching package: 'raster'
#> The following object is masked from 'package:dplyr':
#>
#> select
#> Loading required package: dismo
library(tidyverse)
#> ── Attaching packages
#> ───────────────────────────────────────
#> tidyverse 1.3.2 ──
#> ✔ ggplot2 3.3.6 ✔ purrr 0.3.5
#> ✔ tibble 3.1.8 ✔ stringr 1.4.1
#> ✔ tidyr 1.2.1 ✔ forcats 0.5.2
#> ✔ readr 2.1.3
#> ── Conflicts ────────────────────────────────────────── tidyverse_conflicts() ──
#> ✖ tidyr::extract() masks raster::extract()
#> ✖ dplyr::filter() masks stats::filter()
#> ✖ stringr::fixed() masks recipes::fixed()
#> ✖ dplyr::lag() masks stats::lag()
#> ✖ raster::select() masks dplyr::select()
library(tidymodels)
#> ── Attaching packages ────────────────────────────────────── tidymodels 1.0.0 ──
#> ✔ broom 1.0.1 ✔ tune 1.0.1
#> ✔ dials 1.0.0 ✔ workflows 1.1.0
#> ✔ infer 1.0.3 ✔ workflowsets 1.0.0
#> ✔ modeldata 1.0.1 ✔ yardstick 1.1.0
#> ✔ rsample 1.1.0
#> ── Conflicts ───────────────────────────────────────── tidymodels_conflicts() ──
#> ✖ scales::discard() masks purrr::discard()
#> ✖ tidyr::extract() masks raster::extract()
#> ✖ dplyr::filter() masks stats::filter()
#> ✖ stringr::fixed() masks recipes::fixed()
#> ✖ dplyr::lag() masks stats::lag()
#> ✖ raster::select() masks dplyr::select()
#> ✖ yardstick::spec() masks readr::spec()
#> ✖ recipes::step() masks stats::step()
#> ✖ dials::threshold() masks dismo::threshold()
#> ✖ raster::update() masks recipes::update(), stats::update()
#> • Use tidymodels_prefer() to resolve common conflicts.
library(spatialsample)
data("iberolacerta.clade")
data("euro.worldclim")
monticola <- iberolacerta.clade$species$monticolaWe start by generating a {tidymodels} compatable sf object for the
species we want to model.
dat <- sdm_data(monticola$presence.points,
bg = monticola$range,
n = 5000,
coords = c("Longitude",
"Latitude"),
crs = 4326)
dat
#> Simple feature collection with 5260 features and 2 fields
#> Geometry type: POINT
#> Dimension: XY
#> Bounding box: xmin: -9.31549 ymin: 39.83402 xmax: 0.6627441 ymax: 43.8299
#> Geodetic CRS: WGS 84
#> First 10 features:
#> present pnt_origin pnts
#> 1 present data POINT (-5.171215 43.06957)
#> 2 present data POINT (-6.036635 43.02531)
#> 3 present data POINT (-7.679727 40.38852)
#> 4 present data POINT (-7.790437 40.30959)
#> 5 present data POINT (-7.47334 43.78935)
#> 6 present data POINT (-6.575039 42.9107)
#> 7 present data POINT (-5.132756 43.49572)
#> 8 present data POINT (-7.787378 40.39362)
#> 9 present data POINT (-4.941888 43.3531)
#> 10 present data POINT (-7.621731 40.3417)
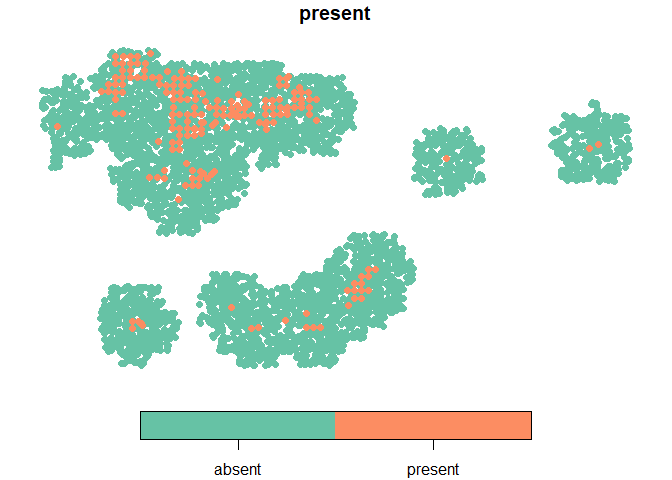
plot(dat %>%
dplyr::select(present) %>%
arrange(present),
pch = 19)Now we can use the {spatialsample} package to create spatial cross
validation folds! We will first create a regular cross validation fold
object for comparison. Note that this can take awhile because a spatial
distance is calculated between all points! We are working on a faster
method for pseudo-absence data.
## regular CV
cv_folds <- vfold_cv(dat, 9)
## spatial CV
cv_folds_spat <- spatial_block_cv(dat, method = "snake",
n = c(6, 4),
v = 9,
buffer = 50000)
cv_folds_spat
#> # 9-fold spatial block cross-validation
#> # A tibble: 9 × 2
#> splits id
#> <list> <chr>
#> 1 <split [3098/902]> Fold1
#> 2 <split [4056/427]> Fold2
#> 3 <split [3923/761]> Fold3
#> 4 <split [4175/517]> Fold4
#> 5 <split [4408/375]> Fold5
#> 6 <split [4501/71]> Fold6
#> 7 <split [4293/210]> Fold7
#> 8 <split [3346/789]> Fold8
#> 9 <split [3246/1207]> Fold9
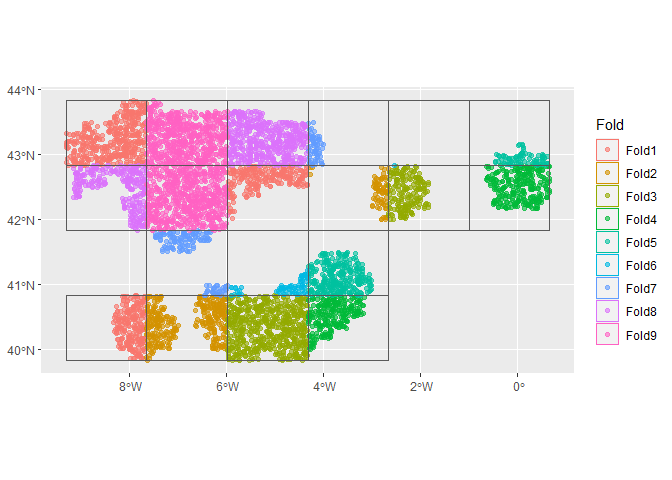
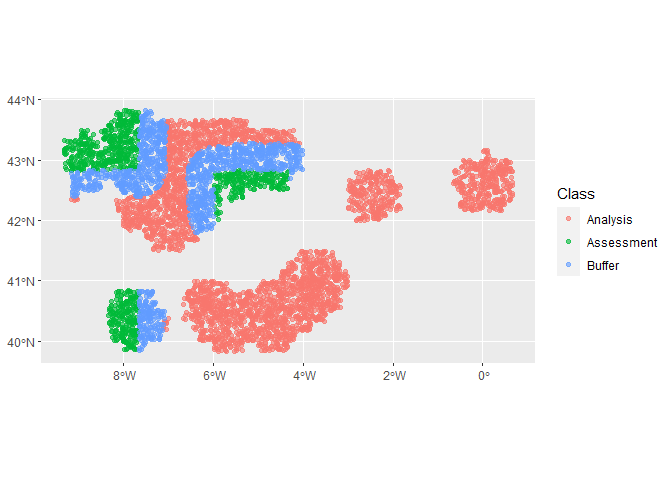
## look at the spatial folds
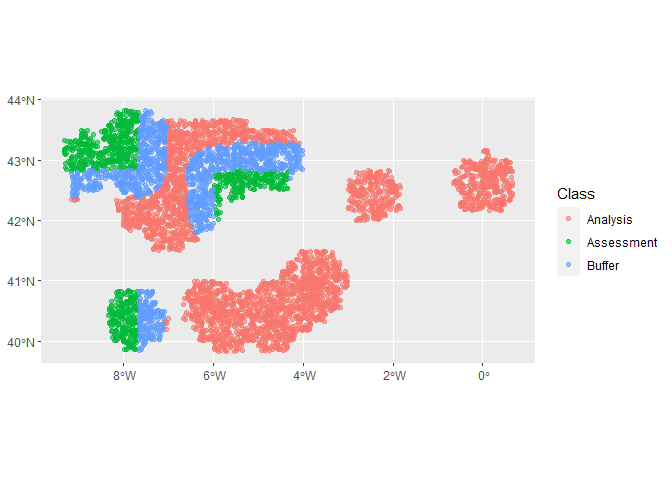
autoplot(cv_folds_spat)autoplot(cv_folds_spat$splits[[1]])autoplot(cv_folds_spat$splits[[8]])Let’s create a recipe to apply some common data processing steps to
prepare for SDM fitting. We start with step_add_env_vars(), which is a
{tidymodels} function. The rest are standard steps from the
{recipes} package. step_impute_median() imputes missing values
(since we will be doing a random forest model which cannot handle
missing values). step_YeoJohnson() does a Yeo-Johnson transformation
on the predictors, which makes them more symmetric and
‘Gaussian-like’. It also saves the parameters used in the
transformation which will be automatically applied to any test data used
for prediction later. Finally step_normalize() transforms predictors
to have a mean of zero and a standard deviation of 1, so that they are
all on the same scale. It also saves the means and sds to be applied to
test data. To see the result of the step we run prep() and
bake(new_data = NULL).
sdm_recipe <- recipe(dat) %>%
step_add_env_vars(env = euro.worldclim) %>%
step_impute_median(all_predictors()) %>%
step_YeoJohnson(all_predictors()) %>%
step_normalize(all_predictors())
test <- prep(sdm_recipe) %>%
bake(NULL)
test
#> # A tibble: 5,260 × 22
#> present pnts pnt_origin bio1 bio2 bio3 bio4
#> <fct> <POINT [°]> <fct> <dbl> <dbl> <dbl> <dbl>
#> 1 present (-5.171215 43.06957) data -1.47 0.167 0.777 -0.332
#> 2 present (-6.036635 43.02531) data -1.53 0.167 0.777 -0.336
#> 3 present (-7.679727 40.38852) data 1.07 -0.332 -0.193 -0.0434
#> 4 present (-7.790437 40.30959) data 0.645 -0.763 -1.16 -0.0264
#> 5 present (-7.47334 43.78935) data 1.23 -1.44 1.76 -1.61
#> 6 present (-6.575039 42.9107) data -1.28 0.0786 0.777 -0.248
#> 7 present (-5.132756 43.49572) data 0.854 -1.35 1.27 -1.33
#> 8 present (-7.787378 40.39362) data 1.07 -0.332 -0.193 -0.0434
#> 9 present (-4.941888 43.3531) data 0.593 -1.35 0.777 -1.27
#> 10 present (-7.621731 40.3417) data -0.644 -1.44 -2.58 0.00321
#> # … with 5,250 more rows, and 15 more variables: bio5 <dbl>, bio6 <dbl>,
#> # bio7 <dbl>, bio8 <dbl>, bio9 <dbl>, bio10 <dbl>, bio11 <dbl>, bio12 <dbl>,
#> # bio13 <dbl>, bio14 <dbl>, bio15 <dbl>, bio16 <dbl>, bio17 <dbl>,
#> # bio18 <dbl>, bio19 <dbl>Now we can setup a random forest model with {parsnip} and combine it
with our recipe using a workflow from the {workflows} package..
mod <- rand_forest() %>%
set_engine("ranger", importance = "impurity") %>%
set_mode("classification")
wf <- workflow() %>%
add_recipe(sdm_recipe) %>%
add_model(mod,
formula = present ~ .)
wf
#> ══ Workflow ════════════════════════════════════════════════════════════════════
#> Preprocessor: Recipe
#> Model: rand_forest()
#>
#> ── Preprocessor ────────────────────────────────────────────────────────────────
#> 4 Recipe Steps
#>
#> • step_add_env_vars()
#> • step_impute_median()
#> • step_YeoJohnson()
#> • step_normalize()
#>
#> ── Model ───────────────────────────────────────────────────────────────────────
#> Random Forest Model Specification (classification)
#>
#> Engine-Specific Arguments:
#> importance = impurity
#>
#> Computational engine: rangerNow we fit the model! We will start with the regular cross validation
folds, and use fit_resamples() to generate metrics based on the fits
to each of the validation sets in our folds. Then collect_metrics()
will calculate the means across folds for us.
fit_1 <- wf %>%
fit_resamples(cv_folds,
control = control_resamples(extract = extract_fit_engine))
fit_1 %>%
collect_metrics()
#> # A tibble: 2 × 6
#> .metric .estimator mean n std_err .config
#> <chr> <chr> <dbl> <int> <dbl> <chr>
#> 1 accuracy binary 0.948 9 0.00311 Preprocessor1_Model1
#> 2 roc_auc binary 0.824 9 0.0121 Preprocessor1_Model1Okay, so our ROC AUC value is pretty good at around 0.82. Now we do the same for the spatial cross validation folds.
fit_2 <- wf %>%
fit_resamples(cv_folds_spat,
control = control_resamples(extract = extract_fit_engine))
#> ! Fold6: internal: No control observations were detected in `truth` with control level 'pre...
#> ! Fold7: internal: No control observations were detected in `truth` with control level 'pre...
fit_2 %>%
collect_metrics()
#> # A tibble: 2 × 6
#> .metric .estimator mean n std_err .config
#> <chr> <chr> <dbl> <int> <dbl> <chr>
#> 1 accuracy binary 0.968 9 0.0127 Preprocessor1_Model1
#> 2 roc_auc binary 0.577 7 0.0541 Preprocessor1_Model1Using spatially independent cross validation folds has shown us our model does much more poorly if we ask it to generalise to spatial areas not in the training set. Now ROC AUC is only ~ 0.58 – considerably worse. Looking at the individual folds, there is substantial variation in the quality of models.
fit_2$.metrics
#> [[1]]
#> # A tibble: 2 × 4
#> .metric .estimator .estimate .config
#> <chr> <chr> <dbl> <chr>
#> 1 accuracy binary 0.955 Preprocessor1_Model1
#> 2 roc_auc binary 0.775 Preprocessor1_Model1
#>
#> [[2]]
#> # A tibble: 2 × 4
#> .metric .estimator .estimate .config
#> <chr> <chr> <dbl> <chr>
#> 1 accuracy binary 0.991 Preprocessor1_Model1
#> 2 roc_auc binary 0.743 Preprocessor1_Model1
#>
#> [[3]]
#> # A tibble: 2 × 4
#> .metric .estimator .estimate .config
#> <chr> <chr> <dbl> <chr>
#> 1 accuracy binary 0.989 Preprocessor1_Model1
#> 2 roc_auc binary 0.503 Preprocessor1_Model1
#>
#> [[4]]
#> # A tibble: 2 × 4
#> .metric .estimator .estimate .config
#> <chr> <chr> <dbl> <chr>
#> 1 accuracy binary 0.983 Preprocessor1_Model1
#> 2 roc_auc binary 0.454 Preprocessor1_Model1
#>
#> [[5]]
#> # A tibble: 2 × 4
#> .metric .estimator .estimate .config
#> <chr> <chr> <dbl> <chr>
#> 1 accuracy binary 0.984 Preprocessor1_Model1
#> 2 roc_auc binary 0.476 Preprocessor1_Model1
#>
#> [[6]]
#> # A tibble: 2 × 4
#> .metric .estimator .estimate .config
#> <chr> <chr> <dbl> <chr>
#> 1 accuracy binary 1 Preprocessor1_Model1
#> 2 roc_auc binary NA Preprocessor1_Model1
#>
#> [[7]]
#> # A tibble: 2 × 4
#> .metric .estimator .estimate .config
#> <chr> <chr> <dbl> <chr>
#> 1 accuracy binary 1 Preprocessor1_Model1
#> 2 roc_auc binary NA Preprocessor1_Model1
#>
#> [[8]]
#> # A tibble: 2 × 4
#> .metric .estimator .estimate .config
#> <chr> <chr> <dbl> <chr>
#> 1 accuracy binary 0.919 Preprocessor1_Model1
#> 2 roc_auc binary 0.437 Preprocessor1_Model1
#>
#> [[9]]
#> # A tibble: 2 × 4
#> .metric .estimator .estimate .config
#> <chr> <chr> <dbl> <chr>
#> 1 accuracy binary 0.894 Preprocessor1_Model1
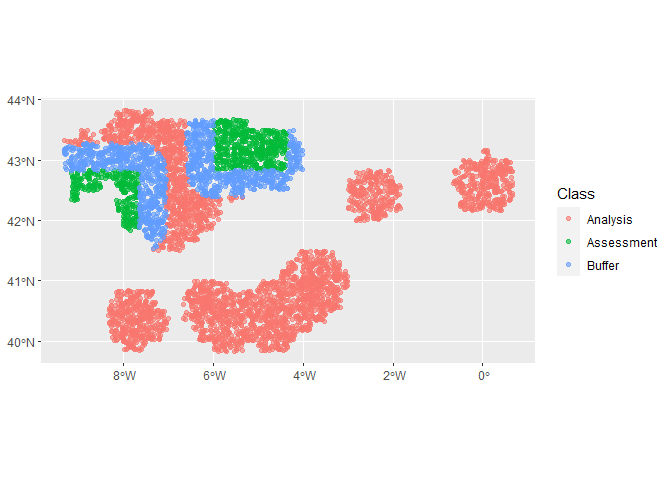
#> 2 roc_auc binary 0.653 Preprocessor1_Model1The first fold the model looks to have done a reasonable job. Which one was that?
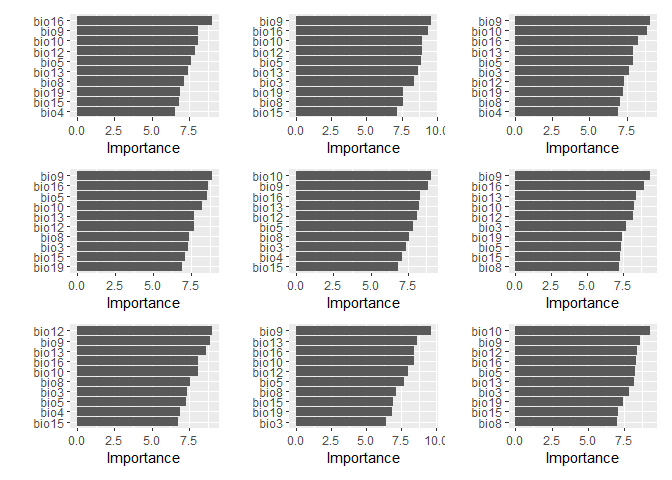
autoplot(cv_folds_spat$splits[[1]])Let’s have a look at the importance values determined by the random forest for our variables.
library(vip)
#>
#> Attaching package: 'vip'
#> The following object is masked from 'package:utils':
#>
#> vi
library(patchwork)
#>
#> Attaching package: 'patchwork'
#> The following object is masked from 'package:raster':
#>
#> area
fit_1 %>%
unnest(.extracts) %>%
pull(.extracts) %>%
map(vip) %>%
wrap_plots(ncol = 3, nrow = 3)The ordering is reasonably consistent between different folds. Now, the spatial folds:
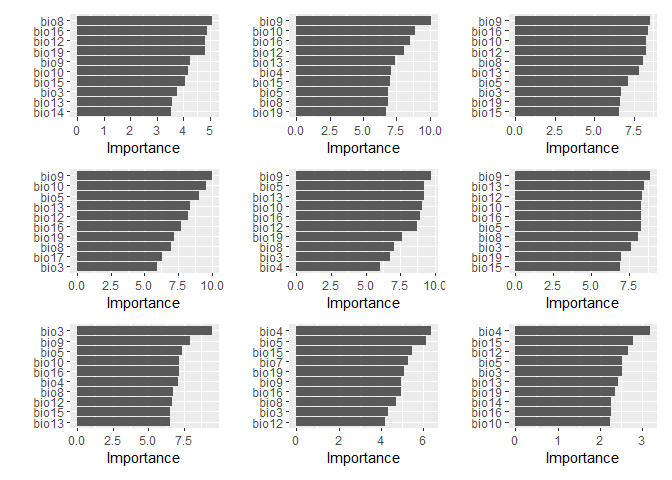
fit_2 %>%
unnest(.extracts) %>%
pull(.extracts) %>%
map(vip) %>%
wrap_plots(ncol = 3, nrow = 3)There seems to be quite a bit more variation in what variables are important between different spatial folds. Which is interesting.
We can try and improve the performance of the model on spatially
independent data by using the spatial cross validation folds to tune the
hyperparameters of the model. We can use the {tune} package for this.
Using tune_bayes() we can use Bayesian optimization to find an optimal
set. What hyperparameters should we tune. For random forest we only have
three, mtry, trees, and min_n. Let’s try tuning all three. First
we make a new model object where we designate these parameters for
tuning, then wrap it into a new workflow.
mod_tune <- rand_forest(mtry = tune(),
trees = tune(),
min_n = tune()) %>%
set_engine("ranger", importance = "impurity") %>%
set_mode("classification")
wf_tune <- workflow() %>%
add_recipe(sdm_recipe) %>%
add_model(mod_tune,
formula = present ~ .)
wf_tune
#> ══ Workflow ════════════════════════════════════════════════════════════════════
#> Preprocessor: Recipe
#> Model: rand_forest()
#>
#> ── Preprocessor ────────────────────────────────────────────────────────────────
#> 4 Recipe Steps
#>
#> • step_add_env_vars()
#> • step_impute_median()
#> • step_YeoJohnson()
#> • step_normalize()
#>
#> ── Model ───────────────────────────────────────────────────────────────────────
#> Random Forest Model Specification (classification)
#>
#> Main Arguments:
#> mtry = tune()
#> trees = tune()
#> min_n = tune()
#>
#> Engine-Specific Arguments:
#> importance = impurity
#>
#> Computational engine: rangerTuning is now as simple as calling tune_bayes(). First we set up an
initial set of tuning models using a tuning grid (regularly spaced
values of the hyper-parameters). By the way, this will take awhile. If
you want to do this on your computer I would recommend setting up a
parallel backend for tuning (see
https://tune.tidymodels.org/articles/extras/optimizations.html).
tune_init <- wf_tune %>%
tune_grid(cv_folds_spat,
grid = 27,
control = control_grid(verbose = interactive()))Now this serves as initial values for tune_bayes().
final_params <- extract_parameter_set_dials(mod_tune) %>%
finalize(dat)
tuned <- wf_tune %>%
tune_bayes(cv_folds_spat,
initial = tune_init,
iter = 50,
param_info = final_params,
control = control_bayes(verbose = interactive(),
no_improve = 50L))tuned %>%
show_best()After all that we haven’t much improved the ability of our model to predict spatially separated testing data sets! This is not that surprising since random forest generally doesn’t need much tuning. And ultimately, the problem is not poor hyper-parameters but overfitting to spatial patterns found in the data. This cannot be prevented except by finding some way to help the model ‘account’ for spatial autocorrelation in the data. There are some approaches to doing this, which might help, but can only go so far. The problem of space is impossible to make go away completely, at least using statistical methods.
Note that the best solutions had a very low mtry. This means the
random forest is only using 1 to 3 variables at a time to make
predictions. This implies all variable contain mostly the same amount of
information and don’t interact much, suggesting that these climate
predictors have little to do with the species distribution, each is
mainly being used for their spatial information. The best bet here, as
in most cases is to find better predictors that are more definitely
related to the species’ known ecology!
Lastly, {tidysdm} makes it easy to generate visualizable predictions
on the original landscape using the create_prediction_grid() function,
which creates a grid of x and y values, optionally with a polygon
attached to help with plotting. Feeding this to the augment function
will automatically make predictions based on the model and the bind
those prediction back to the prediction grid data, ready for plotting.
We will make hexagons which look much cooler than squares (just use
square = FALSE).
pred_grid <- create_prediction_grid(bg = monticola$range, n = 2500, square = FALSE, include_polygons = TRUE)
plot(pred_grid$polygon)Now to make the predictions we first do a final fit of our random forest model on the full data set, and using the best hyper-parameters from our spatial cross validation.
final_fit <- wf_tune %>%
finalize_workflow(select_best(tuned)) %>%
fit(dat)
final_fit## Does not work yet
pred_grid_preds <- final_fit %>%
augment(pred_grid)