Construct a barplot for your STRUCTURE/ADMIXTURE output using starmie in a few lines of code!
# install.packages('starmie')
library(starmie)
str_output <- system.file("extdata/microsat_testfiles/", "locprior_K5.out_f",
package = "starmie")
k5_data <- loadStructure(str_output)
k5_data
#> struct object containing run information for k = 5
#> Model run parameters:
#> individuals : 100
#> loci : 10
#> populations assumed : 5
#> Burn-in period : 10000
#> Reps : 20000
#> turned off : RANDOMIZE
#> Model fit statistics:
#> Estimated Ln Prob of Data : -1408.8
#> Mean value of ln likelihood : -1343
#> Variance of ln likelihood : 131.5
#> Mean value of alpha : 0.0452
#> MCMC diagnostics available: FALSE
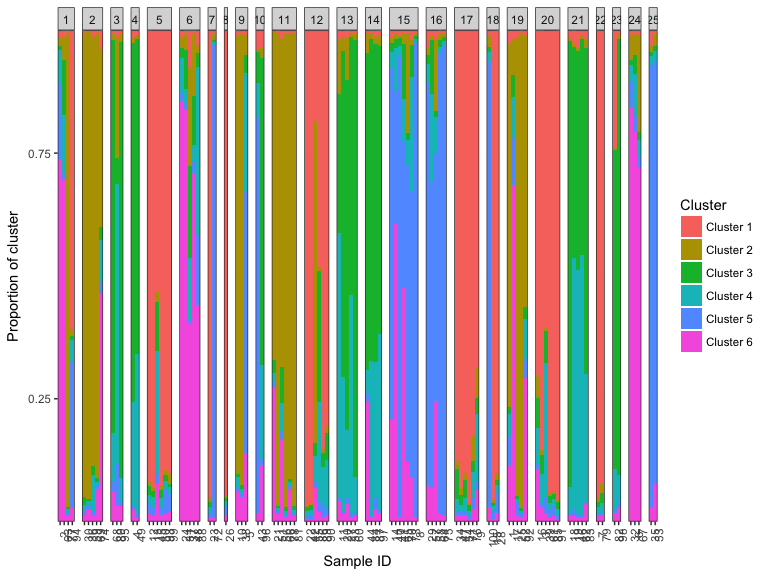
plotBar(k5_data, facet = FALSE)
#> Extracting population labels from STRUCTURE output.A very common part of modern population genetics analysis is inferring underlying population structure from genetic markers such as single nucleotide polymorphisms (SNPs) or microsatellites. The two main methods for this task are the Bayesian STRUCTURE algorithm or the frequentist ADMIXTURE. We have found that processing the output of these programs and performing meaningful inference and visualization of the results is far more difficult than it should be. This is why we wrote starmie.
Some key features:
- Parse output from STRUCTURE or ADMXITURE into a usable data structure in R.
- Plot model diagnostics to perform inference on choice of 'K'.
- Create the 'standard' STRUCTURE bar plot showing cluster memberships.
- Plot model checks such as MCMC chains on the admixture coefficient (\alpha) and the log-likelihood.
- Visualizing output from STRUCTURE analysis
This vignette outlines how to use starmie to do basic tasks after running STRUCTURE at the command line.
To use all the options in starmie for STRUCTURE output, we require for each run the 'out_f' file produced by the program and the logging information so we can produce MCMC diagnostics. To get the latter the output of STRUCTURE must be redirected to a file. Below we present an example of running STRUCTURE in multiple runs for each K in parallel. We assume that the mainparams and extraparams files are correctly specified and that the user has access to the path of the STRUCTURE binary. Also make sure RANDOMIZE option is turned off, so independent seeds can be set in each run.
input_file <- system.file("inst/extdata/microsat_testfiles", "locprior.str",
package = "starmie")
main_params <- system.file("inst/extdata/microsat_testfiles", "mainparams",
package = "starmie")
extra_params <- system.file("inst/extdata/microsat_testfiles", "extraparams",
package = "starmie")
runStructure("path/to/structure", input_file, main_params, extra_params, "run",
5, 2, 2)The basic unit of analysis for starmie is the struct object, which contains the model information in the STRUCTURE out_f file and optionally the logging information for the MCMC diagnostics. As an example, we have run STRUCTURE on simulated microsatellite data from the STRUCTURE example data and save the out_f. To create a struct object from a run we use the following:
library(starmie)
# path to file name
k6_file <- system.file("extdata/microsat_testfiles/", "locprior_K6.out_f", package = "starmie")
# create struct object
k6_msat <- loadStructure(k6_file)
k6_msat
#> struct object containing run information for k = 6
#> Model run parameters:
#> individuals : 100
#> loci : 10
#> populations assumed : 6
#> Burn-in period : 10000
#> Reps : 20000
#> turned off : RANDOMIZE
#> Model fit statistics:
#> Estimated Ln Prob of Data : -1462.4
#> Mean value of ln likelihood : -1333.7
#> Variance of ln likelihood : 257.4
#> Mean value of alpha : 0.0455
#> MCMC diagnostics available: FALSEThe STRUCTURE object contains the following information about a single run:
| attributes | description |
|---|---|
| K | K parameter supplied to STRUCTURE |
| run_params | Input parameters |
| mem_df | Assigned cluster membership proportions |
| allele_freqs | Pairwise Fst values between inferred clusters |
| avg_dist_df | Average nucleotide distance within clusters |
| fst_df | Within cluster average Fst values |
| fit_stats_df | Model fit diagnositcs |
| ancest_df | Individual ancestral probability of membership to cluster |
| clust_allele_list | Estimated ancestral allele frequencies for each cluster |
| burn_df | MCMC burn-in diagnositcs |
| nonburn_df | MCMC post burn-in diagnostics |
Of most interest to users would be the ancest_df which is the Q-matrix of individual cluster membership probabilities. To extract that information for inspection use the helper function getQ.
Q_hat <- getQ(k6_msat)To make the bar-plot simply type:
plotBar(k6_msat, facet = FALSE)
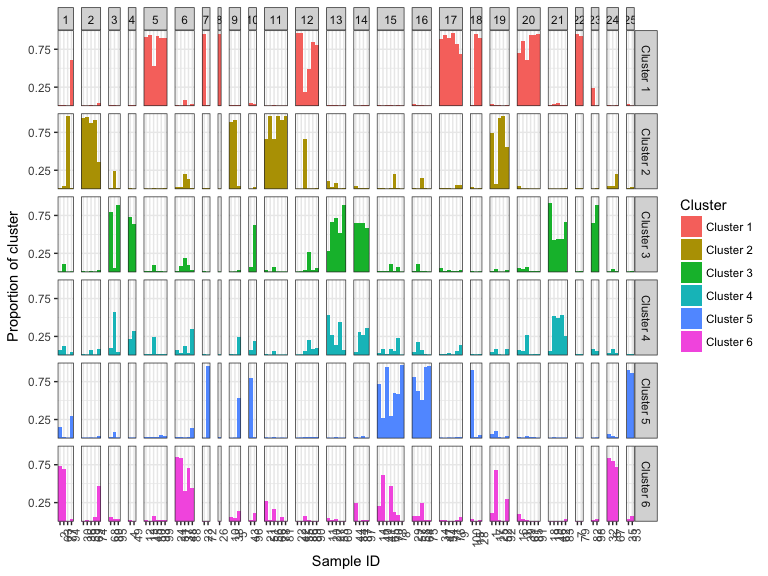
#> Extracting population labels from STRUCTURE output.This will group the known sample labels into population labels if they were supplied to the STRUCTURE run. Alternatively, you can facet the inferred cluster labels to make it easier to see outliers and geographical groupings.
plotBar(k6_msat)
#> Extracting population labels from STRUCTURE output.If you have not given population labels to your samples you can also add them using the populations argument.
The structList is a container for manipulating multiple struct objects. Some potential use-cases are:
- load multiple (K) values from different runs and perform model comparisons.
- pool a sequence of runs over the same value of (K) and perform CLUMPPing
On our example microsatellite data to add the second run for the results of running STRUCTURE (K) = 6 ,we first load the output file, and then pass both struct objects to the structList function.
k6_file_run2 <- system.file("extdata/microsat_testfiles/", "run2_locprior_K6.out_f",
package = "starmie")
k6_run2 <- loadStructure(k6_file_run2)
k6_all <- structList(k6_msat, k6_run2)
k6_all
#> structList object containing 2 STRUCTURE runs.
#> Number of Ks by number of runs:
#> 6
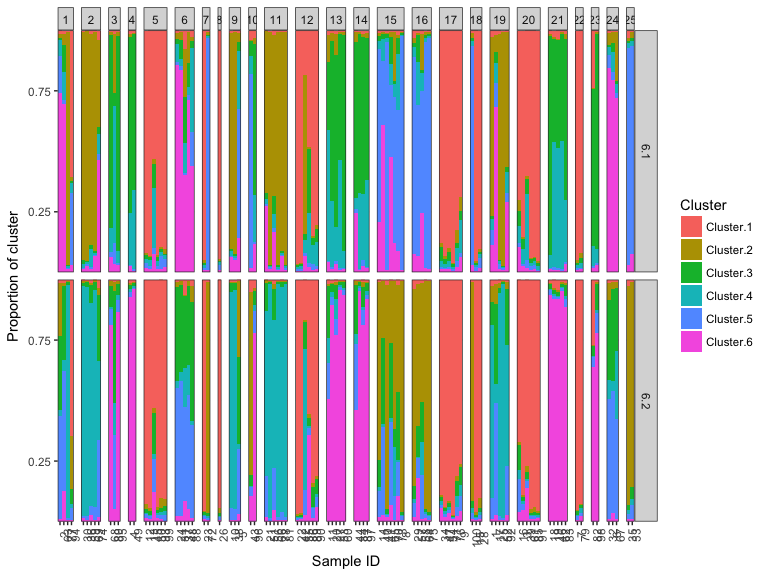
#> 2We can also compare the cluster labeling by using plotMultiK (and see that label-switching over different MCMC runs is a problem!)
plotMultiK(k6_all)
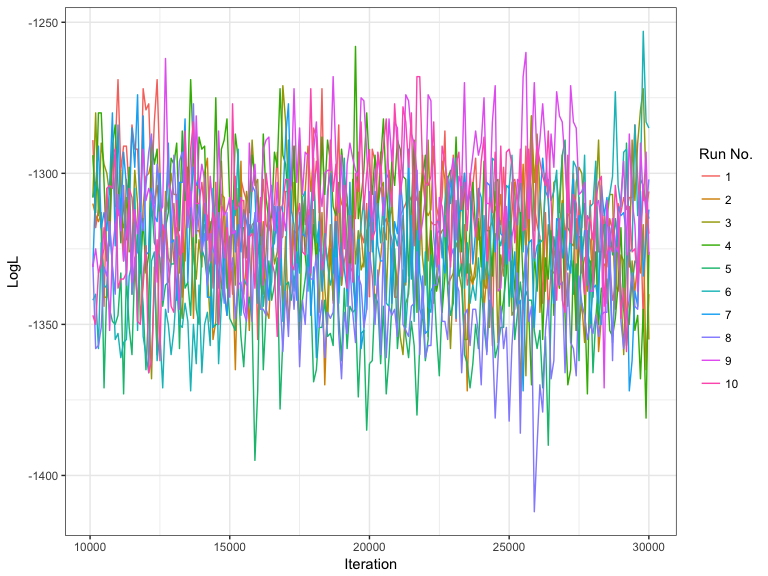
#> Extracting population labels from STRUCTURE output.A very simple approach to determining whether you need to rerun a STRUCTURE a model is to plot the estimated log-likelihood over each iteration over the post burn-in MCMC phase. If the chains have converged the log-likelihood should stabilize towards the final iterations and the variance within a run should be relatively low. The plotMCMC can plot the log-likelihood or admixture coefficient against the iteration over different runs and different (K) values. Note this requires the logging file to be read by loadStructure.
Here we show an example when (K) = 10 and the number of runs is also 10.
multiple_runs_k10 <- exampleStructure("mcmc_diagnostics")
mcmc_out <- plotMCMC(multiple_runs_k10, facet = FALSE)head(mcmc_out$mcmc_info)
#> K Iteration Alpha LogL run
#> 1: 10 10100 0.034 -1289 1
#> 2: 10 10200 0.038 -1318 1
#> 3: 10 10300 0.039 -1312 1
#> 4: 10 10400 0.040 -1314 1
#> 5: 10 10500 0.042 -1344 1
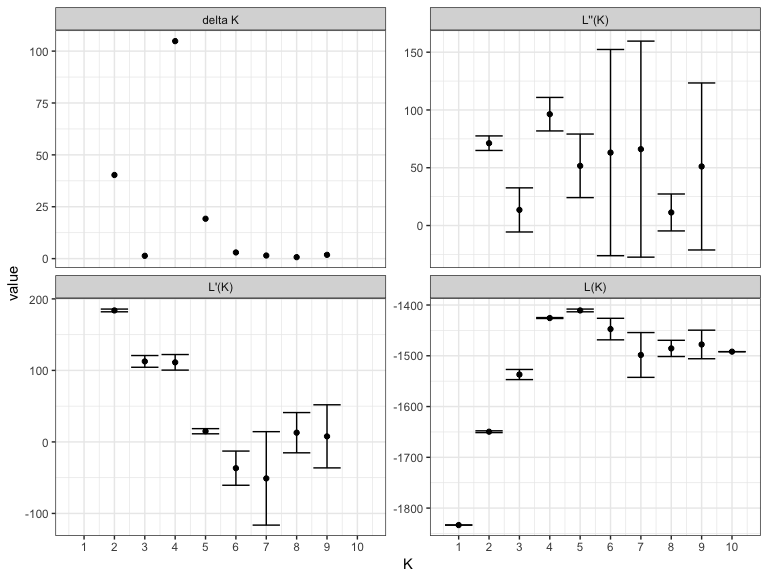
#> 6: 10 10600 0.042 -1305 1Usually you would run STRUCTURE multiple times for multiple values of (K) and then use estimates of the log-likelihood to determine the 'best' choice of (K) that explains the population structure in your data. There are two choices for model selection - either use the maximum mean log-posterior probability estimated by STRUCTURE or use the Evanno method. The bestK function returns the value of (K) that is estimated by these methods and also produces diagnostic plots.
multi_K <- exampleStructure("multiple_runs")
bestK(multi_K)
#> Creating diagnostic plots for structure runs.#> K variable value sd
#> 1 1 L(K) -1833.4000000 0.1414214
#> 2 1 L'(K) NA NA
#> 3 1 L''(K) NA NA
#> 4 1 delta K NA NA
#> 5 2 L(K) -1649.5500000 1.7677670
#> 6 2 L'(K) 183.8500000 1.9091883
#> 7 2 L''(K) 71.2500000 6.2932504
#> 8 2 delta K 40.3050865 NA
#> 9 3 L(K) -1536.9500000 9.9702056
#> 10 3 L'(K) 112.6000000 8.2024387
#> 11 3 L''(K) 13.5000000 19.0918831
#> 12 3 delta K 1.3540343 NA
#> 13 4 L(K) -1425.6500000 0.9192388
#> 14 4 L'(K) 111.3000000 10.8894444
#> 15 4 L''(K) 96.3500000 14.4956890
#> 16 4 delta K 104.8149821 NA
#> 17 5 L(K) -1410.7000000 2.6870058
#> 18 5 L'(K) 14.9500000 3.6062446
#> 19 5 L''(K) 51.6500000 27.5064538
#> 20 5 delta K 19.2221396 NA
#> 21 6 L(K) -1447.4000000 21.2132034
#> 22 6 L'(K) -36.7000000 23.9002092
#> 23 6 L''(K) 63.1000000 89.2368758
#> 24 6 delta K 2.9745625 NA
#> 25 7 L(K) -1498.4000000 44.1234631
#> 26 7 L'(K) -51.0000000 65.3366666
#> 27 7 L''(K) 66.1000000 93.4795165
#> 28 7 delta K 1.4980692 NA
#> 29 8 L(K) -1485.5000000 15.9806133
#> 30 8 L'(K) 12.9000000 28.1428499
#> 31 8 L''(K) 11.3000000 15.9806133
#> 32 8 delta K 0.7071068 NA
#> 33 9 L(K) -1477.7000000 28.1428499
#> 34 9 L'(K) 7.8000000 44.1234631
#> 35 9 L''(K) 51.1000000 72.2663130
#> 36 9 delta K 1.8157365 NA
#> 37 10 L(K) -1491.9000000 0.0000000
#> 38 10 L'(K) NA NA
#> 39 10 L''(K) NA NA
#> 40 10 delta K NA NA
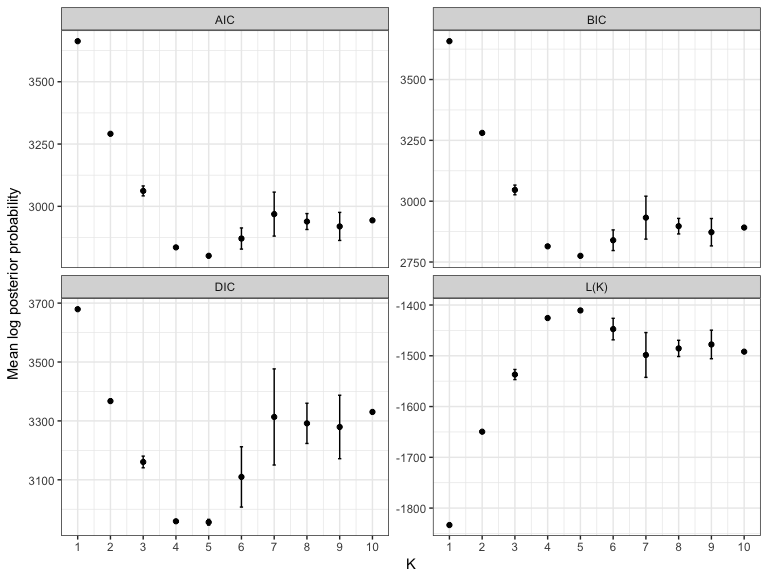
bestK(multi_K, "structure")
#> Creating diagnostic plots for structure runs.
#> K variable value sd
#> 1.1 1 L(K) -1833.400 0.1414214
#> 1.2 1 AIC 3662.800 0.2828427
#> 1.3 1 BIC 3657.590 0.2828427
#> 1.4 1 DIC 3679.300 0.4242641
#> 2.1 2 L(K) -1649.550 1.7677670
#> 2.2 2 AIC 3291.100 3.5355339
#> 2.3 2 BIC 3280.679 3.5355339
#> 2.4 2 DIC 3367.300 5.7982756
#> 3.1 3 L(K) -1536.950 9.9702056
#> 3.2 3 AIC 3061.900 19.9404112
#> 3.3 3 BIC 3046.269 19.9404112
#> 3.4 3 DIC 3160.600 19.7989899
#> 4.1 4 L(K) -1425.650 0.9192388
#> 4.2 4 AIC 2835.300 1.8384776
#> 4.3 4 BIC 2814.459 1.8384776
#> 4.4 4 DIC 2959.200 3.9597980
#> 5.1 5 L(K) -1410.700 2.6870058
#> 5.2 5 AIC 2801.400 5.3740115
#> 5.3 5 BIC 2775.348 5.3740115
#> 5.4 5 DIC 2955.900 9.7580736
#> 6.1 6 L(K) -1447.400 21.2132034
#> 6.2 6 AIC 2870.800 42.4264069
#> 6.3 6 BIC 2839.538 42.4264069
#> 6.4 6 DIC 3109.800 102.3890619
#> 7.1 7 L(K) -1498.400 44.1234631
#> 7.2 7 AIC 2968.800 88.2469263
#> 7.3 7 BIC 2932.328 88.2469263
#> 7.4 7 DIC 3313.400 163.2002451
#> 8.1 8 L(K) -1485.500 15.9806133
#> 8.2 8 AIC 2939.000 31.9612265
#> 8.3 8 BIC 2897.317 31.9612265
#> 8.4 8 DIC 3291.700 68.3065151
#> 9.1 9 L(K) -1477.700 28.1428499
#> 9.2 9 AIC 2919.400 56.2856998
#> 9.3 9 BIC 2872.507 56.2856998
#> 9.4 9 DIC 3279.400 107.7630735
#> 10.1 10 L(K) -1491.900 0.0000000
#> 10.2 10 AIC 2943.800 0.0000000
#> 10.3 10 BIC 2891.697 0.0000000
#> 10.4 10 DIC 3330.400 5.3740115
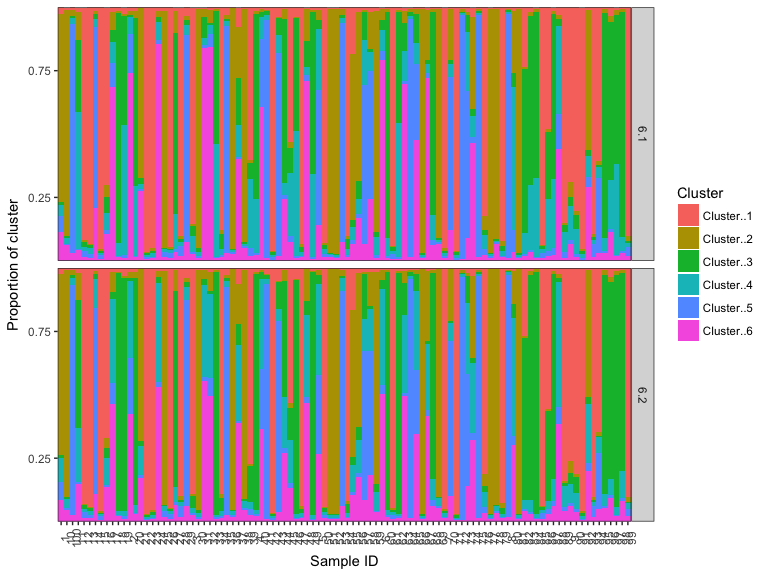
We have written R implementations of the popular CLUMMP and CLUMPAK algorithms for combining Q-matrices over different runs of STRUCTURE. Usually, this step is performed after choosing a value for (K), when the analyst would like to refine their estimates of cluster memberships. To perform CLUMPPING create a structList consisting of the same value of (K) for multiple runs. In each case the Q-matrices and a matrix of column permutations for each run are returned.
We return to the example of our two runs of (K) = 6, stored in k6_all defined above.
Q_list <- lapply(k6_all, getQ)
clumpak_results <- clumpak(Q_list)
clumppy <- clumpp(Q_list, method = "greedy")
# plot the results
plotMultiK(clumppy$Q_list)Several other algorithms to correct label switching are available, including fast implementations for large values of K.
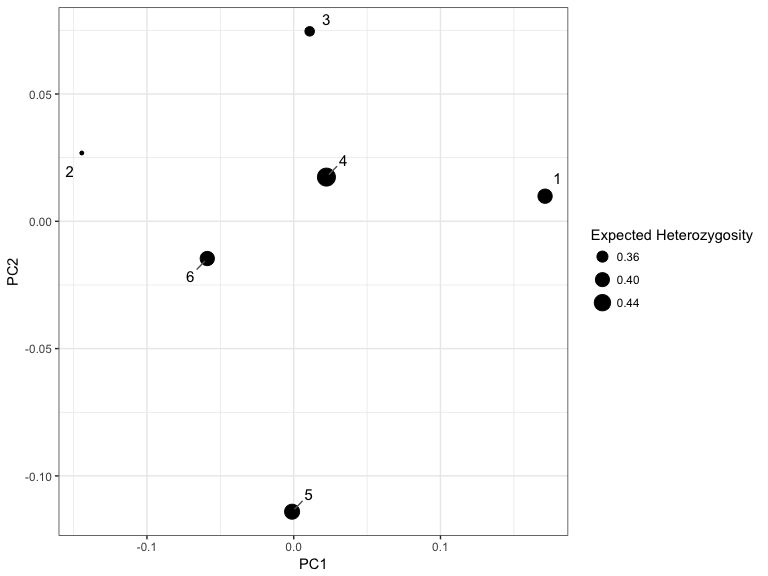
As the STRUCTURE model outputs other information, we have implemented some multidimensional scaling plots to visualize some of the neglected features of the STRUCTURE model.
For example, we can plot the net nucleotide distance between clusters using multidimensional scaling, to the see the relationships between inferred clusters.
plotMDS(k6_msat)Please submit any bugs or feature requests as an issue to https://github.com/sa-lee/starmie/issues
Kopelman, N. M., Mayzel, J., Jakobsson, M., Rosenberg, N. A. & Mayrose, I. Clumpak: a program for identifying clustering modes and packaging population structure inferences across K. Mol. Ecol. Resour. 15, 1179–1191 (2015).
Stephens, M. Dealing with label switching in mixture models. J. R. Stat. Soc. Series B Stat. Methodol. 62, 795–809 (2000).
Jakobsson, M. & Rosenberg, N. A. CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 23, 1801–1806 (2007).
Hubisz, M. J., Falush, D., Stephens, M. & Pritchard, J. K. Inferring weak population structure with the assistance of sample group information. Mol. Ecol. Resour. 9, 1322–1332 (2009).
Falush, D., Stephens, M. & Pritchard, J. K. Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol. Ecol. Notes 7, 574–578 (2007).
Falush, D., Stephens, M. & Pritchard, J. K. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164, 1567–1587 (2003).
Pritchard, J. K., Stephens, M. & Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 155, 945–959 (2000).
Verity, R. & Nichols, R. A. Estimating the Number of Subpopulations (K) in Structured Populations. Genetics (2016).
starmie is available as an R package on CRAN
install.packages("starmie")The development version of starmie can be installed with devtools
install.packages("devtools")
devtools::install_github("sa-lee/starmie")If you would like to also build the vignette with your installation run:
devtools::install_github("sa-lee/starmie", build_vignettes = TRUE)