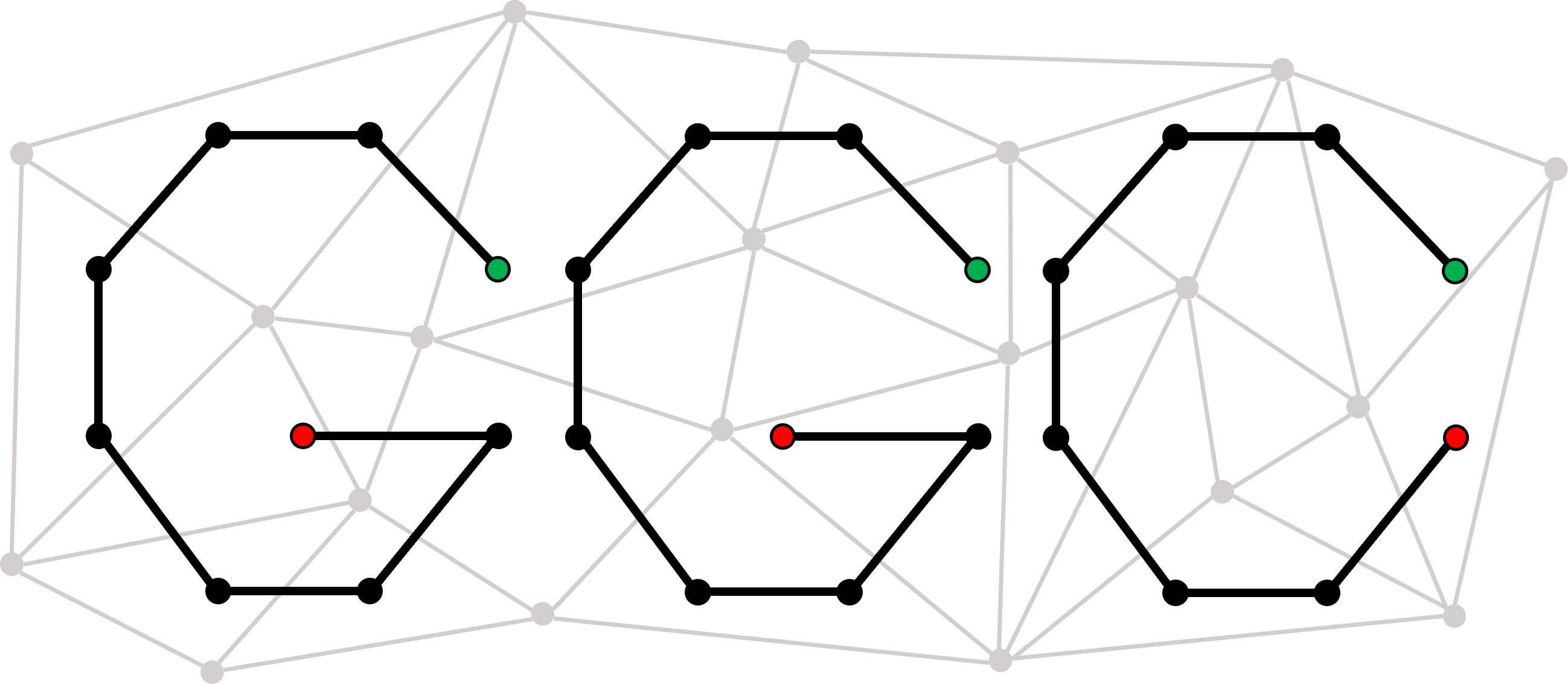
ggCaller traverses Bifrost graphs constructed from bacterial genomes to identify putative gene sequences, known as open reading frames (ORFs).
ggCaller incorporates Balrog to filter ORFs to improve specificity of calls and Panaroo for pangenome analysis and quality control.
Guides for installation, usage and a tutorial can be found here.
ggCaller is available on Linux. If you are running Windows 10/11, Linux can be installed via the Windows Subsystem for Linux (WSL).
We plan to get a MacOS version up and running in the future.
Install through bioconda:
conda install ggcaller
If conda is not installed, first install miniconda, then add the correct channels:
conda config --add channels defaults
conda config --add channels bioconda
conda config --add channels conda-forge
First, install Docker for your OS. If running with WSL2, you should still download Docker Desktop for Windows.
Then pull the latest image::
docker pull samhorsfield96/ggcaller:latest
To run ggCaller, run::
cd test && docker run --rm -it -v $(pwd):/workdir -v $(pwd):/data samhorsfield96/ggcaller:latest ggcaller --balrog-db /app/ggc_db --refs /workdir/pneumo_CL_group2_docker.txt --out /workdir/ggc_out
Required packages and versions can be found in environment_linux.yml and environment_macOS.yml depending on your operating system. In addition, a C++17 compiler (e.g. gcc >=7.3) is required.
For example, using conda (creates ggc_env environment)
conda env create -f environment_linux.yml
conda activate ggc_env
Once all required packages are installed, install ggCaller using:
git clone --recursive https://github.com/samhorsfield96/ggCaller
cd ggCaller
python setup.py install
Please cite the ggCaller pre-print:
Horsfield, S.T., Croucher, N.J., Lees, J.A. "Accurate and fast graph-based pangenome annotation and clustering with ggCaller" bioRxiv 2023.01.24.524926 (2023). doi: https://doi.org/10.1101/2023.01.24.524926
If you use this code, please also cite the dependencies:
- Bifrost: Holley, G., Melsted, P. "Bifrost: highly parallel construction and indexing of colored and compacted de Bruijn graphs." Genome Biol 21(249) (2020). https://doi.org/10.1186/s13059-020-02135-8
- Kseq: seqtk: https://github.com/lh3/seqtk
- SDSL v3:
Succinct Data Structure Library 3.0 <https://github.com/xxsds/sdsl-lite>_
- Balrog: Sommer M.J., Salzberg S.L. "Balrog: A universal protein model for prokaryotic gene prediction." PLoS Comput Biol 17(2): e1008727 (2021). https://doi.org/10.1371/journal.pcbi.1008727
- Eigen v3: Guennebaud, G., Jacob, B. et al. "Eigen v3" (2010). http://eigen.tuxfamily.org
- Boost graph library: Siek, J., Lee, L.Q. & Lumsdaine, A. "Boost graph library" (2002) https://www.boost.org/doc/libs/1_79_0/libs/graph/doc/index.html
- Edlib: Šošić, M., Šikić, M. "Edlib: a C/C++ library for fast, exact sequence alignment using edit distance." Bioinformatics 33(9) (2017). https://doi.org/10.1093/bioinformatics/btw753
- DIAMOND: Buchfink B., Reuter K., Drost H.G. "Sensitive protein alignments at tree-of-life scale using DIAMOND", Nature Methods 18:366–368 (2021). https://doi.org/10.1038/s41592-021-01101-x
- HMMER3: Eddy S.R. "A New Generation of Homology Search Tools Based on Probabilistic Inference." Genome Inform., 23:205-211 (2009).
- MAFFT: Katoh, K., Misawa, K., Kuma, K. & Miyata, T. "MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform." Nucleic Acids Research. 30 (14), 3059–3066 (2002). https://doi.org/10.1093/nar/gkf436
- SNP-sites: Page, A.J., Taylor, B., Delaney, A.J., Soares, J., Seemann, T., Keane, J.A. & Harris, S.R. "SNP-sites: rapid efficient extraction of SNPs from multi-FASTA alignments. Microbial genomics." 2 (4), e000056 (2016). https://doi.org/10.1099/mgen.0.000056
- RapidNJ: Simonsen, M., Pedersen, C. "Rapid computation of distance estimators from nucleotide and amino acid alignments" Proceedings of the ACM Symposium on Applied Computing (2011) https://doi.org/10.1145/1982185.1982208
- Panaroo: Tonkin-Hill, G., MacAlasdair, N., Ruis, C. et al. "Producing polished prokaryotic pangenomes with the Panaroo pipeline." Genome Biol 21(180) (2020). https://doi.org/10.1186/s13059-020-02090-4