- Introduction
- Citations
- File Formats
- Requirements
- Typical Workflow
- Stand-alone Workflow (Old)
- Interactive Visualization of Contacts
- Interactive Visualization of 3D Genomes
- Generation of Contact Matrices
Diploid Chromatin Conformation Capture (Dip-C) reconstructs 3D diploid genomes from single cells by imputing the two chromosome haplotypes linked by each genomic contact.
An alternative (faster and more careful) implementation of the Dip-C algorithm is included in hickit.
Please cite the original Dip-C paper, which described the Dip-C method and algorithm. This work studied a human B-lymphoblastoid cell line (LCL; GM12878), peripheral blood mononuclear cells (PBMCs), and mouse embryonic stem cells (mESCs; raw data from a previous study):
Tan, Longzhi*; Xing, Dong*; Chang, Chi-Han; Li, Heng; Xie, X. Sunney "Three-dimensional genome structures of single diploid human cells," Science 43, 924-928. DOI:10.1126/science.aat5641 (2018).
Our second Dip-C paper included more detailed experimental protocols, the combined use of hickit and this repo, and a more convenient (yet less sensitive) version that uses homemade Nextera instead of multiplex end-tagging amplification (META). This work studied the mouse eye and nose during development, with a focus on rod photoceptors and olfactory sensory neurons (OSNs):
Tan, Longzhi*; Xing, Dong*; Daley, Nicholas; Xie, X. Sunney "Three-dimensional genome structures of single sensory neurons in mouse visual and olfactory systems," Nature Structural & Molecular Biology 26, 297-307. DOI:10.1038/s41594-019-0205-2 (2019).
Our third Dip-C paper included a positive control (purified gDNA), the potential use of low-cost, commerically available Tn5 transposome, multi-channel pipettes, and 384 Nextera barcodes. This work studied the mouse brain (in particular the forebrain: cortex and hippocampus) during postnatal development, and integrated with single-cell transcriptome and other omic data.
Tan, Longzhi†; Ma, Wenping; Wu, Honggui; Zheng, Yinghui; Xing, Dong; Chen, Ritchie; Li, Xiang; Daley, Nicholas; Deisseroth, Karl; Xie, X. Sunney† "Changes in genome architecture and transcriptional dynamics progress independently of sensory experience during post-natal brain development," Cell 184, 1-18. DOI:10.1016/j.cell.2020.12.032 (2021).
- Link
- Protocols:
- Raw data:
- PRJNA679183 (scRNA-seq with MALBAC-DT)
- PRJNA607329 (main Dip-C dataset)
- PRJNA678567 (sensory deprivation Dip-C dataset)
- Processed data: GSE162511
Our fourth Dip-C paper introduced Pop-C and vDip-C.
- Link
- Raw and processed data:
To resolve the two haplotypes, a list of phased single-nucleotide polymorphisms (SNPs) is required in a tab-delimited format (chromosome, coordinate, paternal nucleotide, maternal nucleotide).
An example SNP file for the GM12878 cell line (in hg19 coordinates), based on the 2016-1.0 release of Illumina Platinum Genomes, is provided as snps/NA12878.txt.gz. The first few lines are shown below:
1 534324 G T
1 550515 T C
1 565419 G C
1 655642 G A
1 666543 G A
1 676118 T C
1 705882 A G
1 727529 A G
1 732772 G A
1 742825 A G
The basic output of a chromatin conformation capture (3C/Hi-C) experiment is sequencing reads (or read pairs) containing more than one genomic segments. In each of these reads (sometimes known as chimeric reads), genomic segments far away in the linear genome (or even from different chromosomes) are joined by proximity-based ligation.
These segments are recorded in a .seg file as an intermediate format. Each line represents a read (or read pair) in a tab-delimited format (read name, segment 1, segment 2 ...).
Each segment is recorded as a comma-delimited string: . for read 1 and m (short for "mate") for read 2, start coordinate in the read, end coordinate in the read, chromosome, start coordinate in the genome, end coordinate in the genome, strand of the genome (+ or -), haplotype (. for unknown, 0 for paternal, and 1 for maternal). A segment has a known haplotype if it carries one or more phased SNPs.
An example .seg (short for "segment") file is:
HWI-D00433:595:HLYW7BCXY:1:1209:15116:100489 .,0,211,5,94770308,94770519,+,. m,57,211,5,97167374,97167528,-,0 m,0,61,5,94770541,94770602,-,.
HWI-D00433:595:HLYW7BCXY:1:1113:20520:48210 .,0,193,21,23683758,23683951,-,0 .,188,255,21,25124149,25124216,-,.
HWI-D00433:595:HLYW7BCXY:2:2207:6749:75115 .,39,92,15,71782216,71782269,-,. .,0,43,15,62676681,62676724,+,.
HWI-D00433:595:HLYW7BCXY:1:1103:9994:47614 .,44,278,1,19116987,19117221,+,. .,0,48,1,19108099,19108147,-,.
HWI-D00433:595:HLYW7BCXY:2:1216:14071:49584 .,0,114,11,120680807,120680921,+,. .,109,211,11,120689618,120689720,-,. m,0,211,11,120689228,120689439,+,.
Here I define a "leg" as an endpoint of a read segment, so named because it will form one of the two legs of a chromatin contact. More generally, a leg can be any single point in the genome.
Each leg is recorded as a comma-delimited string: chromosome, coordiante, haplotype.
An example .leg file is:
1,948359,.
1,1192624,.
1,2561820,.
1,2836242,.
1,2954969,1
1,3114198,.
1,3343831,.
1,3455767,.
1,3518062,.
1,3540154,1
Chromatin contacts are a crucial concept in 3C/Hi-C. A contact refers to the proximity-based ligation of two genomic segments far away in the linear genome (or even from different chromosomes). Here I define a contact as the two adjoining endpoints (legs) of two different segments in a same read (or read pair).
Each contact is recorded as a tab-delimited line: leg 1, leg 2.
An example .con (short for "contact") file is:
1,858641,. 1,861338,.
1,861471,. 1,862872,.
1,918037,. 1,1024147,.
1,918249,1 1,1231502,.
1,921617,0 1,956928,.
1,922873,. 1,926783,.
1,923319,. 1,957711,.
1,946196,. 1,1235547,.
1,948480,. 1,1133615,.
1,959161,. 1,962343,.
There are some subtleties in this definition:
- In some studies, contacts are defined only between adjacent segments and therefore corresponds exactly to ligation junctions. When a read (or read pair) contains more than two segments, however, I define contacts between all pairs of segments. This is similar to bulk studies of multi-way contacts.
- The coordinate of a leg is exact (and most likely at a restriction digestion site, for example
GATC) if it resides in the middle of read 1 or read 2. It is approximate if it resides in the unread gap between read 1 and read 2. - The two legs of a contact are interchangeable. To avoid ambiguity, I arbitarily impose that leg 1 < leg 2.
- Here directionalities are ignored. In contrast, hickit preserves this additional information by adopting the
.pairsformat of the 4D Nucleome program as the contact file format.
The primary output of the Dip-C algorithm is the 3D structure of a single-cell genome. Following the definition in nuc_dynamics, the 3D structure of each chromosome is represented by 3D coordinates of regularly spaced points (0 kb, 10 kb, 20 kb, 30 kb ...) along the chromosome. 3D coordinates of points elsewhere will be linearly interpolated from the given points.
Each 3D genome is recorded in a tab-delimited format (chromosome with (pat) for paternal and (mat) for maternal, coordinate, x, y, z).
An example .3dg (short for "3D genome") file is:
1(mat) 1420000 0.791377837067 10.9947291355 -13.1882897693
1(mat) 1440000 -0.268241283699 10.5200875887 -13.0896257278
1(mat) 1460000 -1.3853075236 10.5513787498 -13.1440142173
1(mat) 1480000 -1.55984101733 11.4340829129 -13.6026301209
1(mat) 1500000 -0.770991778399 11.4758488546 -14.5881137222
1(mat) 1520000 -0.0848245107875 12.2624690808 -14.354289628
1(mat) 1540000 -0.458643807046 12.5985791771 -13.4701149287
1(mat) 1560000 -0.810322906201 12.2461643989 -12.3172933413
1(mat) 1580000 -2.08211172035 12.8886838656 -12.8742007778
1(mat) 1600000 -3.52093948201 13.1850935438 -12.4118684428
A .reg (short for "region") file performs a similar role to a BED file, but with haplotype information. This format can be used to exclude regions of copy-number (CN) gains or losses of heterozygosity (LOHs) from a .con file, to set haplotypes in regions of CN losses in a .con file, or to extract regions of interest from a .3dg file.
Each region is recorded as a tab-delimited line: chromosome, haplotype, start coordinate (. for the start of the chromosome), end coordinate (. for the end of the chromosome).
An example .reg file is:
1 . . .
2 1 232800000 .
2 . 238500000 .
4 . . .
5 . 165800000 167800000
5 1 167800000 .
6 1 . .
11 . . .
16 . 40000000 .
19 . . .
Dip-C was tested on Python v2.7.13 (macOS and CentOS), with the following basic requirements:
- NumPy (tested on v1.12.1)
- SciPy (tested on v0.13.3)
Some Dip-C commands have additional requirements:
- Read pre-processing for META (not required for Nextera or other whole-genome amplification methods): pre-meta from pre-pe, which requires seqtk for paired-end reads
- Read alignment: BWA (tested on v0.7.15), SAMtools (tested on v1.3)
- Contact pre-processing & 3D reconstruction: hickit (tested on v0.1.1)
visand other mmCIF scripts: PDBx Python Parser- mmCIF viewing: PyMol
align: rmsd
In our latest work, both the main Dip-C algorithm and 3D modeling are now carried out with hickit, a much faster and more careful implementation. Below is a typical workflow of such combined use of hickit and this repo (with mm10 as an example genome):
# align reads
bwa mem -5SP genome.fa R1.fq.gz R2.fq.gz | gzip > aln.sam.gz # for Nextera
#seqtk mergepe R1.fq.gz R2.fq.gz | pre-meta - | bwa mem -5SP -p genome.fa - | gzip > aln.sam.gz # for META
# extract segments
hickit.js sam2seg -v snp.txt.gz aln.sam.gz | hickit.js chronly -y - | gzip > contacts.seg.gz # for female
#hickit.js sam2seg -v snp.txt.gz aln.sam.gz | hickit.js chronly - | hickit.js bedflt par.bed - | gzip > contacts.seg.gz # for male
# resolve haplotypes via imputation
hickit -i contacts.seg.gz -o - | bgzip > contacts.pairs.gz
hickit -i contacts.pairs.gz -u -o - | bgzip > impute.pairs.gz
# generate 3D structures (with 3 replicates)
for rep in `seq 1 3`
do
hickit -s${rep} -M -i impute.pairs.gz -Sr1m -c1 -r10m -c2 -b4m -b1m -O 1m.${rep}.3dg -b200k -O 200k.${rep}.3dg -D5 -b50k -O 50k.${rep}.3dg -D5 -b20k -O 20k.${rep}.3dg
done
# convert from hickit to dip-c formats, and remove repetitive regions from 3D structures
scripts/hickit_pairs_to_con.sh contacts.pairs.gz
scripts/hickit_impute_pairs_to_con.sh impute.pairs.gz
for rep in `seq 1 3`
do
scripts/hickit_3dg_to_3dg_rescale_unit.sh 20k.${rep}.3dg
dip-c clean3 -c impute.con.gz 20k.${rep}.dip-c.3dg > 20k.${rep}.clean.3dg # remove repetitive (contact-less) regions
done
# align replicate structures and calculate RMSD (overall value in .log file)
dip-c align -o aligned.20k. 20k.[1-3].clean.3dg 2> 20k.align.log > 20k.align.color
# convert to juicebox format for interactive viewing
# raw contacts
java -Xmx2g -jar juicer_tools.jar pre -n contacts.pairs.gz contacts.hic mm10
# haplotype-resolved contacts
scripts/con_imputed_to_juicer_pre_short.sh impute.con.gz
java -Xmx2g -jar juicer_tools.jar pre -n impute.juicer.txt.gz impute.hic color/mm10.chr.hom.len
# calculate single-cell chromatin compartment values along the genome
dip-c color2 -b1000000 -H -c color/mm10.cpg.1m.txt -s contacts.con.gz > cpg_b1m.color2 # contact-based
dip-c color -c color/mm10.cpg.20k.txt -s3 20k.1.clean.3dg > cpg_s3.color # 3D-structure-based
# calculate radial positioning
dip-c color -C 20k.1.clean.3dg > C.color
# color by chromosome number and visualize as mmCIF (viewable with pymol)
dip-c color -n color/mm10.chr.txt 20k.1.clean.3dg | dip-c vis -c /dev/stdin 20k.1.clean.3dg > 20k.1.clean.n.cifThe original workflow, which was before the development of hickit and uses a slightly modified version of nuc_dynamics for 3D modeling, can be found in an archived document.
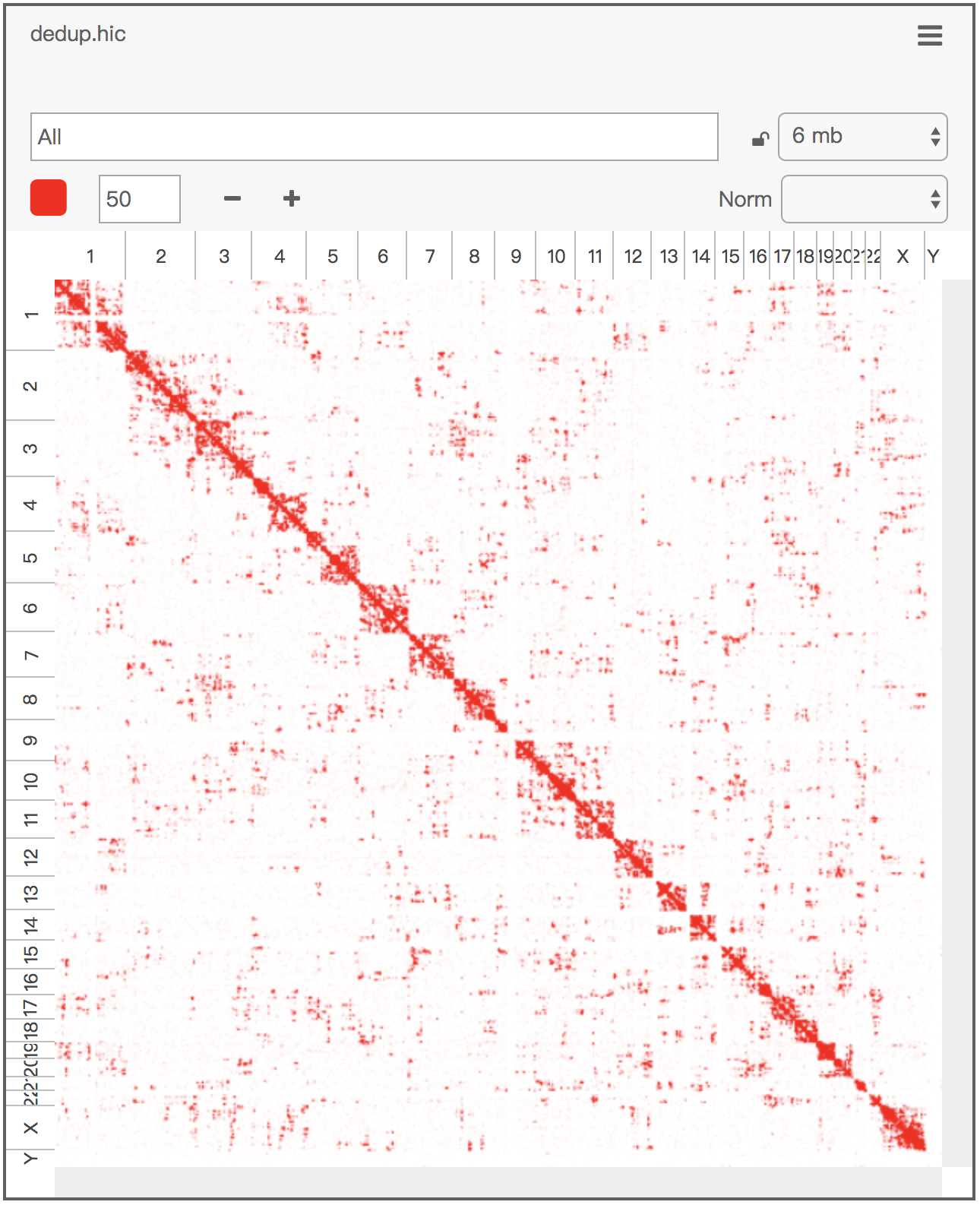
A simple shell script, con_to_juicer_pre_short.sh, converts a .con file into the short format input for Juicer Tools Pre and, subsequently, into a .hic file:
con_to_juicer_pre_short.sh dedup.con.gz # which generates dedup.juicer.txt.gz
java -Xmx2g -jar juicer_tools.jar pre -n dedup.juicer.txt.gz dedup.hic hg19Alternatively, another shell script, con_imputed_to_juicer_pre_short.sh, works on an imputed (haplotype-resolved) .con file:
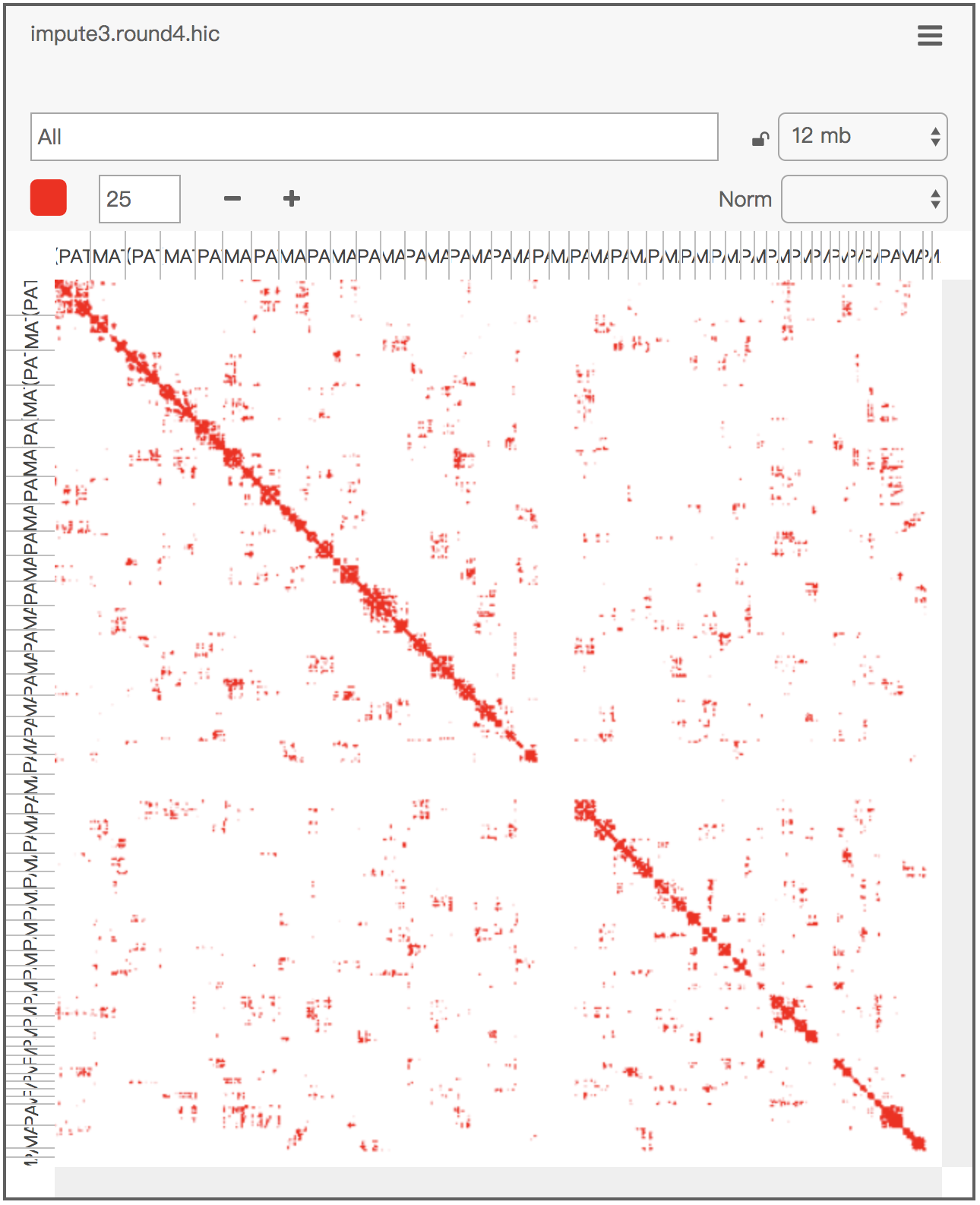
con_imputed_to_juicer_pre_short.sh impute3.round4.con.gz # which generates impute3.round4.juicer.txt.gz
java -Xmx2g -jar juicer_tools.jar pre -n impute3.round4.juicer.txt.gz impute3.round4.hic color/hg19.chr.hom.lenThe output .hic file can then be viewed interactively in Juicebox (the error message about the lack of normalization can be ignored).
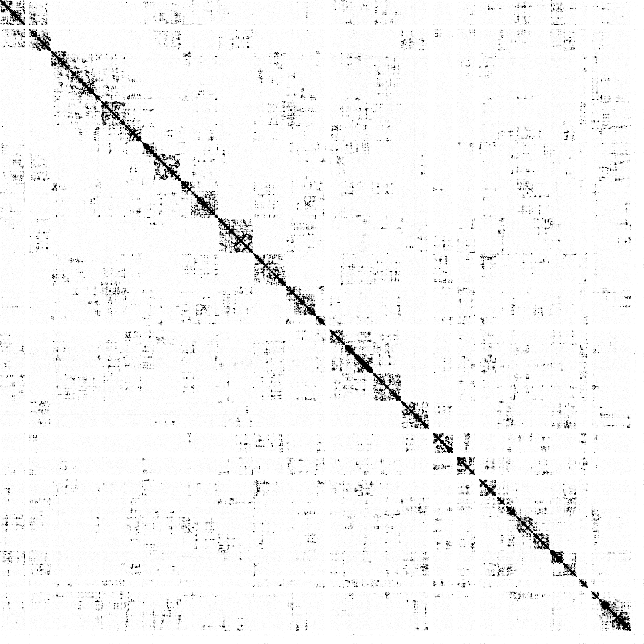
Below is the visualization of an example .con file:
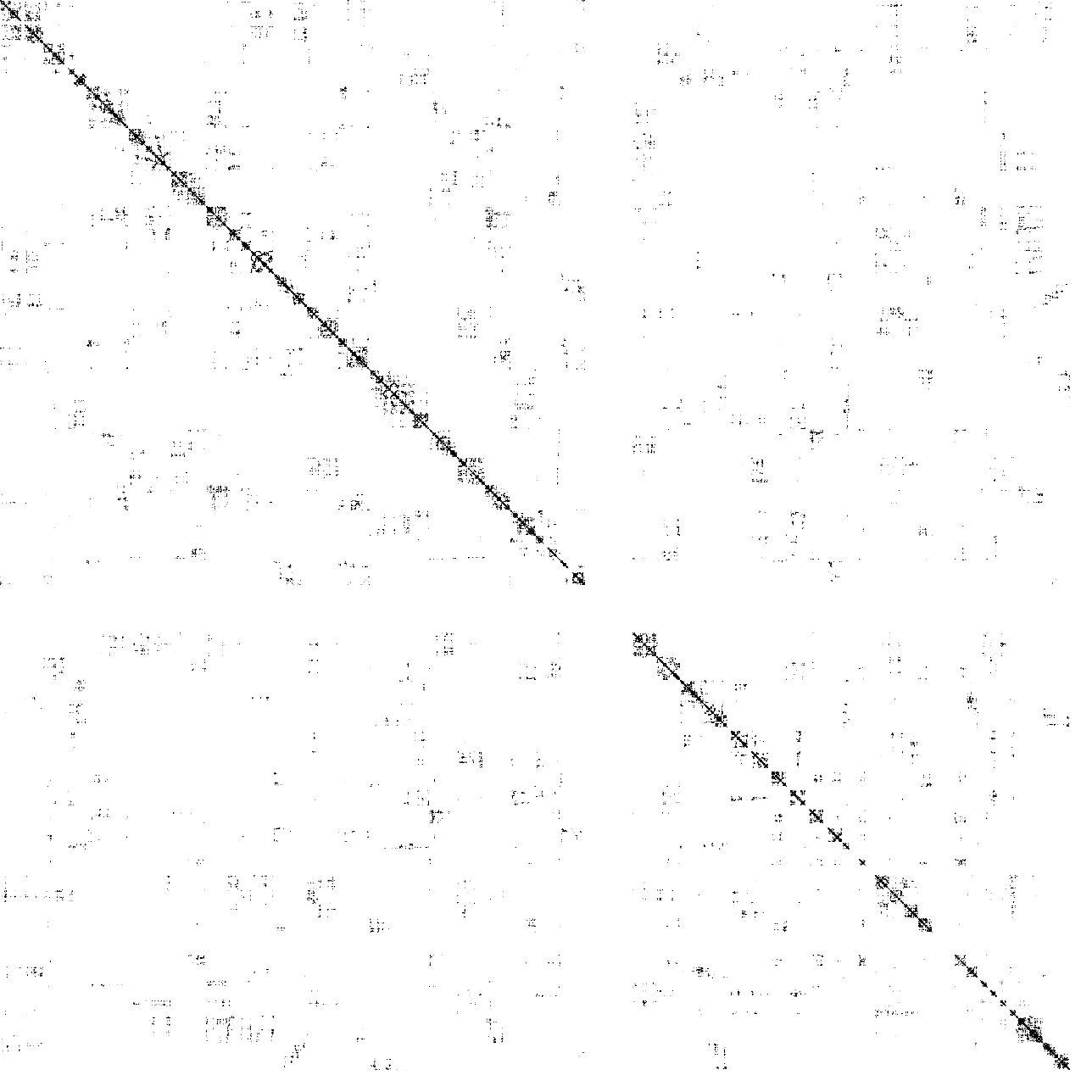
Below is the visualization of an example imputed .con file:
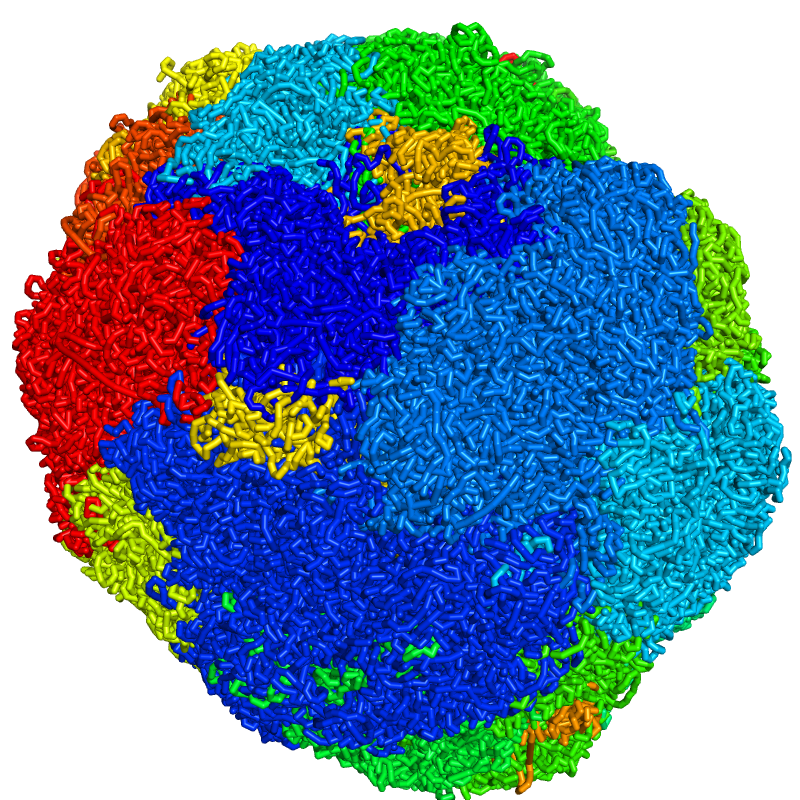
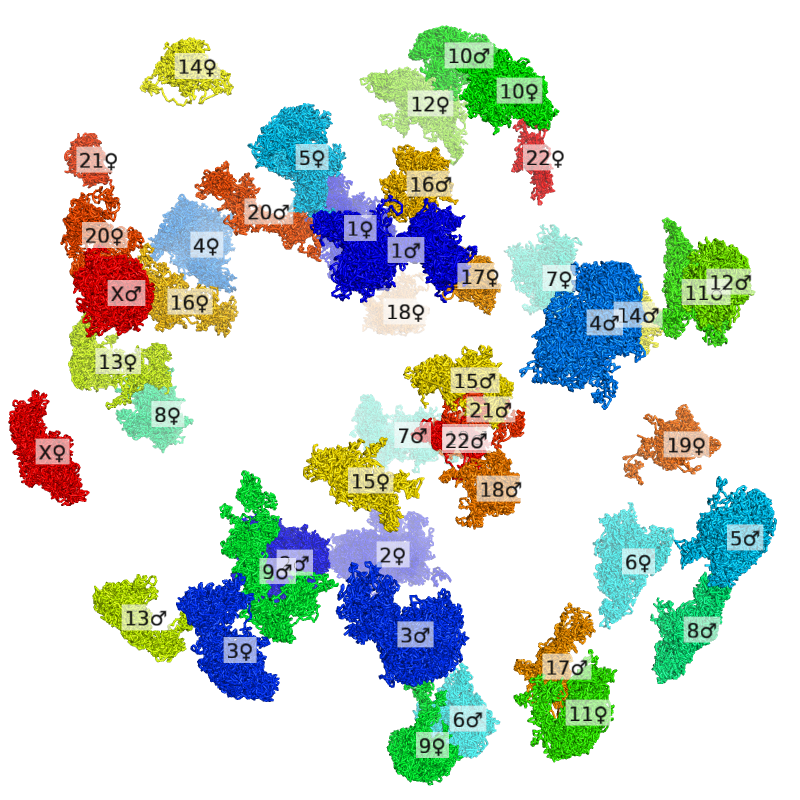
We will get started with a simple example: visualize a single cell colored by chromosome (rainbow with blue = chromosome 1 and red = chromosome X/Y).
Before viewing, the following line must be added to the start-up script (.pymolrc) of PyMol. Otherwise, PyMol may create bonds between numerous pairs of particles, consuming a large amount of CPU and memory.
set connect_mode, 4First, the .3dg file is colored by chromosome with dip-c color -n and converted into a .cif file with dip-c vis, which takes a minute:
dip-c color -n color/hg19.chr.txt cell.3dg | dip-c vis -c /dev/stdin cell.3dg > cell.n.cifThe resulting file, cell.n.cif, can now be dragged into an opened PyMol window, and styled as follows:
viewport 800, 800
set ray_shadows,0
as sticks, all
set_bond stick_radius, 0.5, all
spectrum b, rainbow, all, 1, 23The image can be stored as a .png file by running:
png cell.n.png, 800, 800, ray=1Below is the final image:
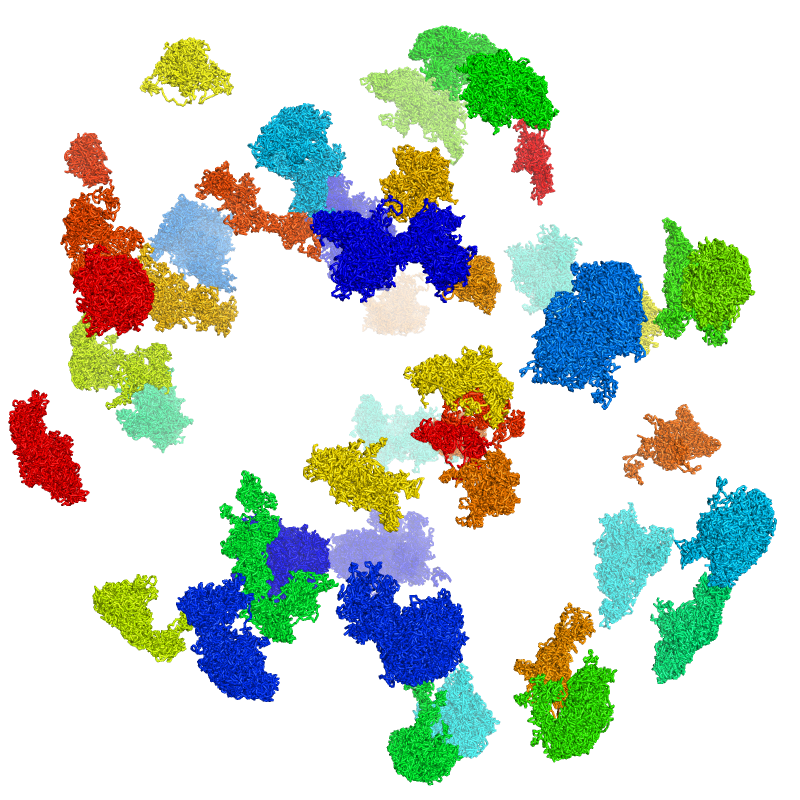
Sometimes it is desirable to move chromosomes apart, and in some cases to label each one, for better visualization. Chromosomes can be moved apart with dip-c exp:
dip-c exp cell.3dg > cell.exp.3dg 2> cell.exp.pyThe main output file, cell.exp.3dg, can now be colored and converted:
dip-c color -n color/hg19.chr.txt cell.exp.3dg | dip-c vis -c /dev/stdin cell.exp.3dg > cell.exp.n.cifIn PyMol, this .cif can be styled and printed in the same way as above. Below is the image:
A movie of rotating the nucleus and moving the chromosomes apart, if desired, can be generated by replacing part of pymol/pymol_movie_exp.py with the corresponding part of the secondary output file of dip-c exp, cell.exp.py. In particular, after running the PyMol scripts, we can go to the menu option File -> Export Movie As -> MPEG..., and select "Ray (slow)" (or "Draw (fast)" to save time; difference in video quality is negligible), "ffmpeg" as the encoder, and "MPEG 4".
An example movie, generated from this file in the GEO database with pymol/pymol_movie_exp.py, can be found in this tweet.
To label each chromosome, we first need to store the current camera position in PyMol:
get_viewBelow is an example printout:
### cut below here and paste into script ###
set_view (\
0.707233906, 0.091803670, -0.700993419,\
-0.206852838, 0.975012839, -0.081004508,\
0.676041245, 0.202291712, 0.708551943,\
0.000000000, 0.000000000, -1053.545166016,\
0.212142944, 0.067672729, 0.137741089,\
830.623046875, 1276.467285156, -20.000000000 )
### cut above here and paste into script ###Going back to the original .3dg file, we will shrink each chromosome into a single particle after moving them:
dip-c exp -c cell.3dg > cell.exp_c.3dg
dip-c vis cell.exp_c.3dg | sed 's/(mat)/♀/g; s/(pat)/♂/g' > cell.exp_c.cifThis new .cif file can be dragged into another PyMol window and styled with the above get_view printout:
viewport 800, 800
set_view (\
0.707233906, 0.091803670, -0.700993419,\
-0.206852838, 0.975012839, -0.081004508,\
0.676041245, 0.202291712, 0.708551943,\
0.000000000, 0.000000000, -1053.545166016,\
0.212142944, 0.067672729, 0.137741089,\
830.623046875, 1276.467285156, -20.000000000 )
set ray_shadows,0
hide all
label all, chain
set label_size, 10
set label_bg_color, white
set label_bg_transparency, 0.4
png cell.exp.label.png, 800, 800, ray=1The output image can then be overlaid onto the previous image to label each chromosome. Below is the final overlay:
We begin by calculating the CpG frequency of each 20 kb bin along the human genome with cpg.sh, which requires bedtools and takes a while:
cpg.sh genome.fa 20000 > hg19.cpg.20k.txtSome precomputed files are provided. For example, the first few lines of color/hg19.cpg.20k.txt are:
1 20000 0.0279
1 40000 0.0082
1 60000 0.00725
1 80000 0.00715
1 100000 0.0072
1 120000 0.00585
1 140000 0.01945
1 160000 0.00995
1 180000 0.0138889
1 220000 0.0162602
This file can then be used to color a cell with dip-c color -c:
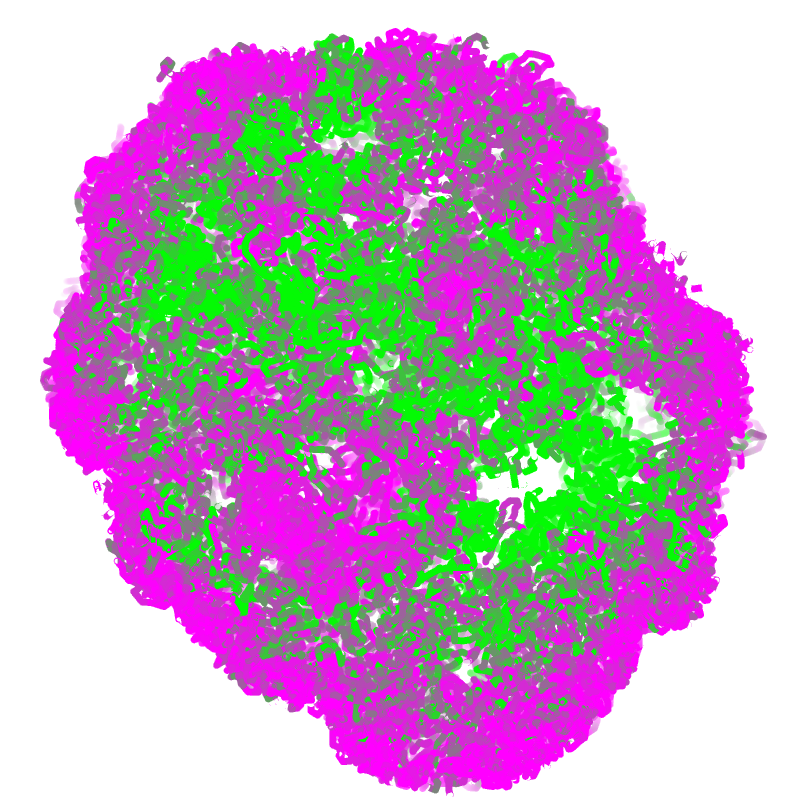
dip-c color -c color/hg19.cpg.20k.txt cell.3dg | dip-c vis -M -c /dev/stdin cell.3dg > cell.cpg.cifThe resulting .cif file can be styled in PyMol to show a single slice, whose coloring requires the spectrumany plugin:
viewport 800, 800
clip slab, 10
set ray_shadows,0
set ambient, 1
set specular, off
set ray_opaque_background, off
as sticks, all
set_bond stick_radius, 0.5, all
spectrumany b, magenta green, all, 0.005, 0.02
png cell.cpg.png, 800, 800, ray=1Note that the cell can be serially sliced by moving the clipping planes:
clip move, 15Below is an example image:
Although originally designed for diploid cells, Dip-C can also handle haploid cells with minor modifications. Specifically, a haploid .3dg file (for example, generated by hickit) will have chromosome names such as 1 instead of 1(mat) and 1(pat). To visualize such file, a dummy haplotype (such as (mat)) must be appended to all chromosome names.
Note that for commonly used haploid mouse embryonic stem cells (mESCs), this dummy value (mat) is in fact accurate, because they are typically generated from oocytes. In contrast, this dummy value is only a placeholder for other haploid cells such as the human eHAP cell line.
In some cases, it might be desirable to convert a .con file into a genome-wide contact matrix. This can be achieved with dip-c bincon. For example, the code below generates a matrix with 5-Mb bins:
dip-c bincon -b 5000000 -H -l color/hg19.chr.len dedup.con.gz > dedup.bincon.txt
dip-c bincon -i -b 5000000 -H -l color/hg19.chr.len . > dedup.bincon.infoThe primary output file, dedup.bincon.txt, contains the contact matrix:
The secondary output file, dedup.bincon.info, contains the genomic cooridinates of all bin centers. The first few lines are:
1 0
1 5000000
1 10000000
1 15000000
1 20000000
1 25000000
1 30000000
1 35000000
1 40000000
1 45000000
Similarly, an imputed (haplotype-resolved) .con file can be converted to a contact matrix with the following code:
dip-c bincon -b 5000000 -l color/hg19.chr.len impute3.round4.con.gz > impute3.round4.bincon.txt
dip-c bincon -i -b 5000000 -l color/hg19.chr.len . > impute3.round4.bincon.infoThe primary output file contains the contact matrix:
The first few lines of the secondary output file are:
1(pat) 0
1(pat) 5000000
1(pat) 10000000
1(pat) 15000000
1(pat) 20000000
1(pat) 25000000
1(pat) 30000000
1(pat) 35000000
1(pat) 40000000
1(pat) 45000000