The process described below can be used for guided de novo genome assembly of SARS-CoV-2 using Illumina paired-end sequencing and the Paragon Genomics CleanPlex® SARS-CoV-2 Panel in samples extracted from patients in Greece.
It is assumed that the obtained coverage is more that the actual coverage required to obtain the full genome sequence of SARS-CoV-2 and that the samples have to be downsampled in order to satisfy the assumptions of most assemblers. As there is already a reference genome for SARS-CoV-2, we will also use this to estimate the requred coverage to obtain one full contig representing the de novo assembled genome of the virus.
Briefly, we are applying the following steps, assuming that we are starting from raw demultiplexed FASTQ files:
- Adapter trimming
- Filtering of low quality reads (if any)
- Given one sample, we perform normalization at different depths.
- For each depth, we align the normalized FASTQ files to the reference genome.
- We visualize the results.
- For each depth, we are performing de novo genome assembly using SPAdes.
- We assess the quality of each SPAdes assembly using BLAT.
- We assess the quality of each SPAdes assembly using QUAST.
- Having estimated the adequate coverage, we normalize all samples to the desired depth
- We perform assembly with SPAdes
- We assess each assembly using QUAST
- If we have incomplete assemblies, we are trying to complete them with Medusa
This section lists the software required to perform the de novo genome assembly of the virus under investigation (SARS-CoV-2) from paired-end Illumina reads. Installation instructions can be found to the page of each software. Most recent versions are preferred.
- FastQC
- cutadapt
- Trim Galore!
- BBNorm
- bwa
- samtools
- bedtools
- SPAdes
- QUAST
- MeDuSa
- MUMmmer
- Jim Kent UCSC tools
- Integrative Genome Browser
- pigz parallel file compressor
Some of these packages are demanding in terms of prerequisites. Maybe the most tricky one is Medusa which depends on MUMmer and Biopython. However, the latest Biopython version does not support Python 2.7 so if you wish to stick to Python 2.7, then you must explicitly install Biopython 1.76:
pip install biopython==1.76
After that, MUMmer must become available to the PATH so either
export PATH=$PATH:/path/to/mummer/executable
or attach /path/to/mummer/executable to the PATH variable in ~/.bashrc:
echo PATH=$PATH:/path/to/mummer/executable >> ~/.bashrc
source ~/.bashrc
De novo genome assembly is a process very sensitive to sequencing errors, as few errors may significantly alter the quality of the end-product. Therefore, a rather strict quality control procedure must be followed to ensure the required quality or to reject samples of very low quality. In this section we describe the steps performed to check the quality of the raw sequencing data and decide if any actions are required to be performed to improve quality prior to assembly.
Firstly, we run a first round of QC using FastQC to determine if any actions need to be taken. The following bash script can be used as a template:
#!/bin/bash
HOME_PATH=/PATH/TO/ANALYSIS/DIRECTORY
FASTQ_PATH=$HOME_PATH/fastq
FASTQ_PATTERN=*.fastq.gz
FASTQC_COMMAND=/PATH/TO/FastQC/fastqc
FASTQC_OUTPUT=$HOME_PATH/fastqc
CORES=8
if [ ! -d $FASTQC_OUTPUT ]
then
mkdir -p $FASTQC_OUTPUT
fi
$FASTQC_COMMAND --outdir $FASTQC_OUTPUT --threads $CORES $FASTQ_PATH/$FASTQ_PATTERN
From the results of FastQC, a lot of useful information may be revealed. Some examples include:
- The presence of adapters
- The presence of bias in the 3'/5' end of reads
- Poor quality in the 3'/5' end of reads
- Poor quality for certain samples
- Sequence over-representation other than adapters
In our case, we see points (1) and (5) above. Point (5) can be discarded due to very high coverage.
After a first round of inspection, we need to improve the quality of the overall dataset prior to continuing with other actions regarding the assembly. Trim Galore! is a good option for this as it automates many processes for you, including standard adapter automated removal and taking care of paired-end reads.
As later assembly tools (SPAdes in our case) also perform read error correction, we will use a soft filtering strategy and then let SPAdes do the rest of the error corrections. Also by default, Trim Galore! is not very aggressive.
A template bash script to wrap Trim Galore! follows. With comments, below the main command, an alternative more strict filtering approach:
#!/bin/bash
HOME_PATH=/PATH/TO/ANALYSIS/DIRECTORY
FASTQ_PATH=$HOME_PATH/fastq
TRIMGALORE_OUTPUT=$HOME_PATH/fastq_qual
TRIMGALORE_COMMAND=/PATH/TO/TrimGalore/trim_galore
CORES=8
if [ ! -d $TRIMGALORE_OUTPUT ]
then
mkdir -p $TRIMGALORE_OUTPUT
fi
for FILE in $FASTQ_PATH/*_R1_001.fastq.gz
do
BASE=`basename $FILE | sed s/_R1_001\.fastq\.gz//`
echo "Processing $BASE"
mkdir -p $TRIMGALORE_OUTPUT/$BASE
F1=$FASTQ_PATH/$BASE"_R1_001.fastq.gz"
F2=$FASTQ_PATH/$BASE"_R2_001.fastq.gz"
$TRIMGALORE_COMMAND \
--length 50 \
--output_dir $TRIMGALORE_OUTPUT/$BASE \
--path_to_cutadapt /opt/ngstools/cutadapt/cutadapt \
--cores $CORES \
--paired \
--retain_unpaired \
--fastqc \
--trim-n $F1 $F2
mv $TRIMGALORE_OUTPUT/$BASE/$BASE"_R1_001_val_1.fq.gz" \
$TRIMGALORE_OUTPUT/$BASE/$BASE"_R1_TG.fastq.gz"
mv $TRIMGALORE_OUTPUT/$BASE/$BASE"_R2_001_val_2.fq.gz" \
$TRIMGALORE_OUTPUT/$BASE/$BASE"_R2_TG.fastq.gz"
mv $TRIMGALORE_OUTPUT/$BASE/$BASE"_R1_001_val_1_fastqc.html" \
$TRIMGALORE_OUTPUT/$BASE/$BASE"_R1_fastqc.html"
mv $TRIMGALORE_OUTPUT/$BASE/$BASE"_R1_001_val_1_fastqc.zip" \
$TRIMGALORE_OUTPUT/$BASE/$BASE"_R1_fastqc.zip"
mv $TRIMGALORE_OUTPUT/$BASE/$BASE"_R2_001_val_2_fastqc.html" \
$TRIMGALORE_OUTPUT/$BASE/$BASE"_R2_fastqc.html"
mv $TRIMGALORE_OUTPUT/$BASE/$BASE"_R2_001_val_2_fastqc.zip" \
$TRIMGALORE_OUTPUT/$BASE/$BASE"_R2_fastqc.zip"
mv $TRIMGALORE_OUTPUT/$BASE/$BASE"_R1_001.fastq.gz_trimming_report.txt" \
$TRIMGALORE_OUTPUT/$BASE/$BASE"_R1_trimming_report.txt"
mv $TRIMGALORE_OUTPUT/$BASE/$BASE"_R2_001.fastq.gz_trimming_report.txt" \
$TRIMGALORE_OUTPUT/$BASE/$BASE"_R2_trimming_report.txt"
if [ -f $TRIMGALORE_OUTPUT/$BASE/$BASE"_R1_001_unpaired_1.fq.gz" ]
then
zcat $TRIMGALORE_OUTPUT/$BASE/$BASE"_R1_001_unpaired_1.fq.gz" \
$TRIMGALORE_OUTPUT/$BASE/$BASE"_R2_001_unpaired_2.fq.gz" \
| pigz > $TRIMGALORE_OUTPUT/$BASE/$BASE"_S_TG.fastq.gz"
rm $TRIMGALORE_OUTPUT/$BASE/$BASE"_R1_001_unpaired_1.fq.gz" \
$TRIMGALORE_OUTPUT/$BASE/$BASE"_R2_001_unpaired_2.fq.gz"
fi
done
For paired-end reads, Trim Galore! produces four outputs: - Mate 1 reads passing QC - Mate 2 reads passing QC (and matched to mate 1) - Mate 1 failed reads - Mate 2 failed reads
We have concatenated all the failed reads to one file to be later fed to the assembly pipelines.
Although TrimGalore! works very well, we may feel safer running also the suggested workflow Paragon Genomics which uses cutadapt but does not perform any quality trimming. Therefore, we apply cutadapt after the Trim Galore! process. It should be noted that quality trimming is a very important step as when we tried to perform de novo assembly by using the Paragon Genomics recommendation alone, SPAdes not only did not manage to assemble the SARS-CoV-2 genome at the tested coverages (20x, 50x, 100x, 200x) but the assembled contig coverage was also very low.
A template bash script to wrap the suggested cutadapt run after Trim Galore! follows:
#!/bin/bash
HOME_PATH=/PATH/TO/ANALYSIS/DIRECTORY
FASTQ_PATH=$HOME_PATH/fastq
CUTADAPT_OUTPUT=$FASTQ_PATH
CUTADAPT_COMMAND=/PATH/TO/cutadapt/cutadapt
CORES=8
for BASE in `ls $FASTQ_PATH`
do
echo "Processing $BASE"
F1=$FASTQ_PATH/$BASE/$BASE"_R1_TG.fastq.gz"
F2=$FASTQ_PATH/$BASE/$BASE"_R2_TG.fastq.gz"
$CUTADAPT_COMMAND \
-a AGATCGGAAGAGCACACGTCTGAA \
-g CCTACACGACGCTCTTCCGATCT \
-A AGATCGGAAGAGCGTCGTGTAGG \
-G TTCAGACGTGTGCTCTTCCGATCT \
-e 0.1 \
-O 9 \
-m 20 \
-n 2 \
-j 8 \
-o $CUTADAPT_OUTPUT/$BASE/$BASE"_R1.fastq.gz" \
-p $CUTADAPT_OUTPUT/$BASE/$BASE"_R2.fastq.gz" $F1 $F2 > \
$CUTADAPT_OUTPUT/$BASE/cutadapt_report.txt
done
For paired-end reads, cutadapt produces two outputs: - Trimmed mate 1 reads - Trimmed mate 2 reads
In this guide, we are not going to use the reads that lost their mate after Trim Galore! processing, although for larger assembly projects, it is recommended that they are used as assemblers use them to support the process.
In this section we take one sample and normalize at a few different depths. We are using the reference genome to align, visualize and inspect the results and we are performing de novo assembly for each depth. We assess the quality using QUAST (and optionally BLAT in UCSC Genome Browser).
We are donwloading and indexing the SARS-CoV-2 reference genome from UCSC. The following commands can be used:
HOME_PATH=/PATH/TO/ANALYSIS/DIRECTORY
REF_PATH=$HOME_PATH/reference
BWA_COMMAND=/PATH/TO/bwa/bwa
mkdir -p $REF_PATH
CWD=`pwd`
cd $REF_PATH
wget http://hgdownload.soe.ucsc.edu/goldenPath/wuhCor1/bigZips/wuhCor1.fa.gz
pigz -d wuhCor1.fa.gz
$BWA_COMMAND index wuhCor1.fa
cd $CWD
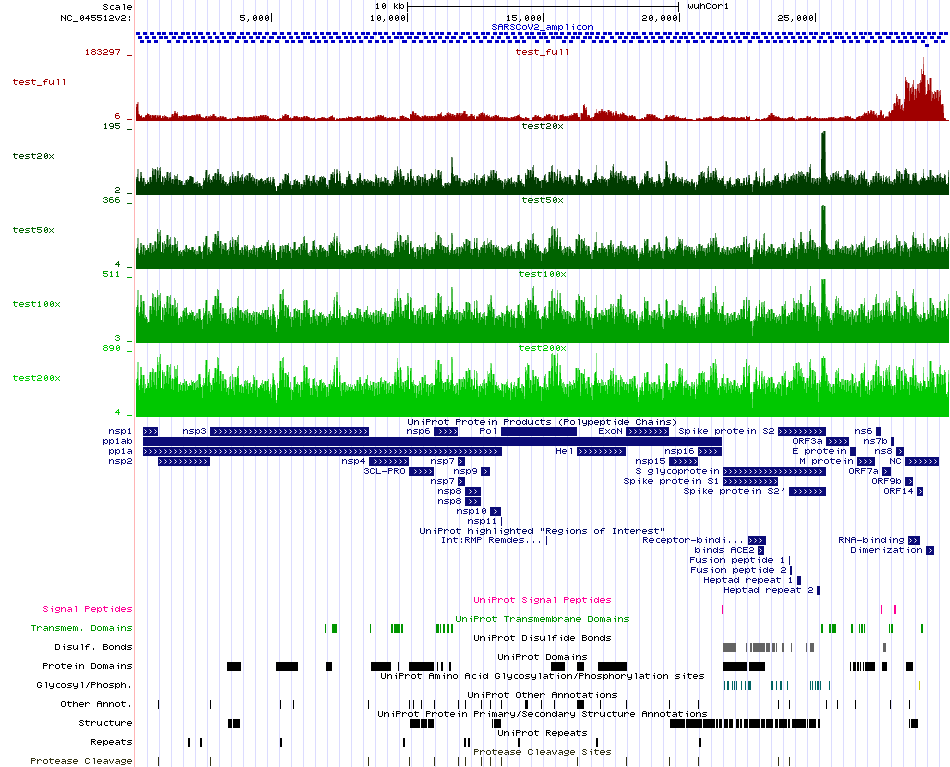
We are using the first sample (alphabetically) for the steps to be followed below. This sample is called 10_S51. For the coverage normalization of the processed FASTQ file we are using the BBNorm suite and the bbnorm.sh tool. Here, we are testing four different desired coverage levels, namely 20x, 50x, 100x and 200x. For each normalized case we are then mapping the resulting normalized FASTQ file to the existing SARS-CoV-2 reference genome to later produce a signal visualization in BigWig format in order to assess the effectiveness of the coverage normalization process by BBNorm. While initially the signal is not uniform across the SARS-CoV-2 genome and there are areas with large coverage differences, BBNorm manages to produce a satisfying uniform coverage result. De nove genome assemblers work much better if the coverage is uniform and the depth is reasonable.
Using the results from BBNorm, we perform de novo genome assembly with SPAdes and we assess each assembly with QUAST. Since we have a reference genome, the assessment is more comprehensible. QUAST produces a very detailed HTML report with various metrics that can be used to assess and improve the assembly.
All the aforementioned process can be accomplished with the following template script:
#!/bin/bash
HOME_PATH=/PATH/TO/ANALYSIS/DIRECTORY
FASTQ_PATH=$HOME_PATH/fastq_qual
REFERENCE_PATH=$HOME_PATH/reference
BBNORM_TEST_OUTPUT=$HOME_PATH/bbnorm_test
GENOME_INDEX=$REFERENCE_PATH/wuhCor1.fa
BBNORM_COMMAND=/PATH/TO/bbmap/bbnorm.sh
BWA_COMMAND=/PATH/TO/bwa/bwa
SAMTOOLS_COMMAND=/PATH/TO/samtools/samtools
BEDTOOLS_COMMAND=/PATH/TO/bedtools/bedtools
SPADES_COMMAND=/PATH/TO/spades/spades.py
QUAST_COMMAND=/PATH/TO/quast/quast.py
KENT_HOME=/PATH/TO/kent
CORES=8
if [ ! -d $BBNORM_TEST_OUTPUT ]
then
mkdir -p $BBNORM_TEST_OUTPUT
fi
BASE=`ls $FASTQ_PATH | head -1`
for DP in 20 50 100 200
do
echo "Processing desired depth $DP"
CURRENT_OUTPATH=$BBNORM_TEST_OUTPUT/$DP"x"
mkdir -p $CURRENT_OUTPATH
F1=$FASTQ_PATH/$BASE/$BASE"_R1.fastq"
F2=$FASTQ_PATH/$BASE/$BASE"_R2.fastq"
pigz -d $F1".gz" $F2".gz"
$BBNORM_COMMAND \
in=$F1 \
in2=$F2 \
out=$CURRENT_OUTPATH/$BASE"_R1.fastq" \
out2=$CURRENT_OUTPATH/$BASE"_R2.fastq" \
min=5 \
target=$DP \
fixspikes=t \
threads=$CORES
pigz $F1 $F2
$BWA_COMMAND mem \
-t $CORES \
$GENOME_INDEX \
$CURRENT_OUTPATH/$BASE"_R1.fastq" \
$CURRENT_OUTPATH/$BASE"_R2.fastq" | \
$SAMTOOLS_COMMAND view -bS -o $BBNORM_TEST_OUTPUT/$DP"x"/$BASE".uns"
$SAMTOOLS_COMMAND sort \
$CURRENT_OUTPATH/$BASE".uns" \
-o $CURRENT_OUTPATH/$BASE".bam"
# Extract genome size for bedGraphToBigWig
$SAMTOOLS_COMMAND view \
-H $CURRENT_OUTPATH/$BASE".bam" | \
grep '^@SQ' | sed s/@SQ\\tSN:// | sed s/\\tLN:/\\t/ > \
$CURRENT_OUTPATH/genome.size
$BEDTOOLS_COMMAND genomecov -bg \
-ibam $CURRENT_OUTPATH/$BASE".bam" | sort -k1,1 -k2g,2 > \
$CURRENT_OUTPATH/$BASE".bedGraph"
$KENT_HOME/bedGraphToBigWig $CURRENT_OUTPATH/$BASE".bedGraph" \
$CURRENT_OUTPATH/genome.size $CURRENT_OUTPATH/$BASE".bigWig"
rm $CURRENT_OUTPATH/$BASE".uns" $CURRENT_OUTPATH/$BASE".bedGraph"
#echo "track type=bigWig name=$BASE_$DPx color=100,100,100 visibility=full maxHeightPixels=128:64:16 bigDataUrl=$BIGDATAURL/$BASE.bigWig" >> \
# $BBNORM_TEST_OUTPUT/tracks.txt
$SPADES_COMMAND \
-1 $CURRENT_OUTPATH/$BASE"_R1.fastq" \
-2 $CURRENT_OUTPATH/$BASE"_R2.fastq" \
-o $CURRENT_OUTPATH/spades \
--threads $CORES --careful
$QUAST_COMMAND \
-r $GENOME_INDEX \
-o $CURRENT_OUTPATH/quast \
$CURRENT_OUTPATH/spades/scaffolds.fasta
pigz $CURRENT_OUTPATH/$BASE"_R1.fastq" $CURRENT_OUTPATH/$BASE"_R2.fastq"
done
In order to visualize the resulting gene signals The resulting BigWig files must be put in a folder served by a web server such as Apache. Then they can be visualized in the UCSC Genome Browser by constructing track lines. Local solutions such as IGV can be used if a web server is not available.
In this section we are normalizing each sample to 100x coverage using BBNorm as before followed by SPAdes assembly. Then we are using QUAST to assess each assembly. For the assemblies that are not complete, we are usng MeDuSa with the reference genome. We can also use one of the assembled genomes to complete it as it would be more appropriate to use one of our local genomes as they are specific to the population examined. However, in practice there are no differences.
The following template script can be used for the aforementioned processes:
#!/bin/bash
HOME_PATH=/PATH/TO/ANALYSIS/DIRECTORY
FASTQ_PATH=$HOME_PATH/fastq_qual
REFERENCE_PATH=$HOME_PATH/reference
ASSEMBLY_PATH=$HOME_PATH/assembly
GENOME_INDEX=$REFERENCE_PATH/wuhCor1.fa
BBNORM_COMMAND=/PATH/TO/bbmap/bbnorm.sh
SPADES_COMMAND=/PATH/TO/spades/spades.py
QUAST_COMMAND=/PATH/TO/quast/quast.py
CORES=8
if [ ! -d $ASSEMBLY_PATH ]
then
mkdir -p $ASSEMBLY_PATH
fi
for BASE in `ls $FASTQ_PATH`
do
echo "Processing $BASE"
CURRENT_OUTPATH=$ASSEMBLY_PATH/$BASE
mkdir -p $CURRENT_OUTPATH
F1=$FASTQ_PATH/$BASE/$BASE"_R1.fastq"
F2=$FASTQ_PATH/$BASE/$BASE"_R2.fastq"
pigz -d $F1".gz" $F2".gz"
$BBNORM_COMMAND \
in=$F1 \
in2=$F2 \
out=$CURRENT_OUTPATH/$BASE"_R1.fastq" \
out2=$CURRENT_OUTPATH/$BASE"_R2.fastq" \
min=5 \
target=100 \
fixspikes=t \
deterministic=t \
threads=$CORES
pigz $F1 $F2
$SPADES_COMMAND \
-1 $CURRENT_OUTPATH/$BASE"_R1.fastq" \
-2 $CURRENT_OUTPATH/$BASE"_R2.fastq" \
-o $CURRENT_OUTPATH/spades \
--threads $CORES --careful
$QUAST_COMMAND \
-r $GENOME_INDEX \
-o $CURRENT_OUTPATH/quast \
$CURRENT_OUTPATH/spades/scaffolds.fasta
pigz $CURRENT_OUTPATH/$BASE"_R1.fastq" $CURRENT_OUTPATH/$BASE"_R2.fastq"
done
Now, by processing QUAST output, we can make a table of how well our assembly process performed overall. Note that if we get many samples with incomplete genomes, we can repeat the process with a normalization to 200x or even more coverage.
The following script template can be used to process QUAST output:
#!/bin/bash
HOME_PATH=/PATH/TO/ANALYSIS/DIRECTORY
ASSEMBLY_PATH=$HOME_PATH/assembly
if [! -d $HOME_PATH/scripts/files ]
then
mkdir -p $HOME_PATH/scripts/files
fi
SPADES_OUT_100x="stats_100x.txt"
printf "%s\t%s\t%s\t%s\t%s\t%s\t%s\t%s\n" "name" "number of contigs" \
"largest contig length" "GC (%)" "N50" "L50" "wuhCor1 match (%)" > \
$SPADES_OUT_100x
for BASE in `ls $ASSEMBLY_PATH`
do
echo "Processing $BASE..."
QUAST_100x=$ASSEMBLY_PATH/$BASE/quast/report.txt
# names
printf "%s\t" $BASE >> $SPADES_OUT_100x
# # contigs
printf "%d\t" `cat $QUAST_100x | head -16 | tail -1 | \
awk '{print $3}'` >> $SPADES_OUT_100x
# length of largest contig
printf "%d\t" `cat $QUAST_100x | head -17 | tail -1 | \
awk '{print $3}'` >> $SPADES_OUT_100x
# GC content
printf "%s\t" `cat $QUAST_100x | head -20 | tail -1 | \
awk '{print $3"%"}'` >> $SPADES_OUT_100x
# N50
printf "%d\t" `cat $QUAST_100x | head -22 | tail -1 | \
awk '{print $2}'` >> $SPADES_OUT_100x
# L50
printf "%d\t" `cat $QUAST_100x | head -26 | tail -1 | \
awk '{print $2}'` >> $SPADES_OUT_100x
# wuhCor1 match (%)
printf "%s\n" `cat $QUAST_100x | head -39 | tail -1 | \
awk '{print $4"%"}'` >> $SPADES_OUT_100x
done
We can perform some basic exploration of the results for 100x coverage in R:
library(ggplot2)
stats100 <- read.delim("files/stats_100x.txt", check.names = FALSE)
summary(stats100)
## name number of contigs largest contig length GC (%)
## 1_S1 : 1 Min. : 1.0 Min. : 2794 38.00% :31
## 10_S51 : 1 1st Qu.: 1.0 1st Qu.:15058 38.01% :18
## 11_S36 : 1 Median : 2.0 Median :25986 37.99% :12
## 13_S24 : 1 Mean : 2.6 Mean :22689 38.02% : 7
## 14_S25 : 1 3rd Qu.: 3.0 3rd Qu.:29868 37.98% : 5
## 16_S45 : 1 Max. :20.0 Max. :30052 37.97% : 3
## (Other):79 (Other): 9
## N50 L50 wuhCor1 match (%)
## Min. : 1424 Min. :1.000 99.883%:38 Mode:logical
## 1st Qu.:15058 1st Qu.:1.000 99.886%: 5 NA's:85
## Median :25986 Median :1.000 99.843%: 4
## Mean :22271 Mean :1.271 99.813%: 3
## 3rd Qu.:29868 3rd Qu.:1.000 99.826%: 2
## Max. :30052 Max. :7.000 99.846%: 2
## (Other):31# Boxplot of number of contigs
stats100$coverage <- rep("100x", nrow(stats100))
ggplot(data = stats100) + geom_boxplot(aes(x = coverage, y = `number of contigs`,
color = coverage), outlier.size = 2) + geom_jitter(aes(x = coverage, y = `number of contigs`,
color = coverage), size = 0.5) + scale_y_continuous(breaks = seq(0, max(stats100$`number of contigs`),
by = 2))Based on the results, we can see that while we have a great match with the SARS-CoV-2 reference genome, quite some samples fail to assemble into one main contig, while nevertheless spanning the virus genome, as can be seen from the average percentage.
Based on the first pass results, we can extend our normalization and assembly script to span 200x and 300x coverages to see if we can get better overall results.
#!/bin/bash
HOME_PATH=/PATH/TO/ANALYSIS/DIRECTORY
ASSEMBLY_PATH=$HOME_PATH/assembly
if [! -d $HOME_PATH/scripts/files ]
then
mkdir -p $HOME_PATH/scripts/files
fi
STATS_OUT="stats.txt"
printf "%s\t%s\t%s\t%s\t%s\t%s\t%s\t%s\n" "name" "number of contigs" \
"largest contig length" "GC (%)" "N50" "L50" "wuhCor1 match (%)" \
"coverage" > $STATS_OUT
for X in `ls $ASSEMBLY_PATH`
do
echo "Processing $X..."
for BASE in `ls $ASSEMBLY_PATH/$X`
do
echo " Processing $BASE..."
QUAST_REPORT=$ASSEMBLY_PATH/$X/$BASE/quast/report.txt
# names
printf "%s\t" $BASE >> $STATS_OUT
# # contigs
printf "%d\t" `cat $QUAST_REPORT | head -16 | tail -1 | \
awk '{print $3}'` >> $STATS_OUT
# length of largest contig
printf "%d\t" `cat $QUAST_REPORT | head -17 | tail -1 | \
awk '{print $3}'` >> $STATS_OUT
# GC content
printf "%s\t" `cat $QUAST_REPORT | head -20 | tail -1 | \
awk '{print $3"%"}'` >> $STATS_OUT
# N50
printf "%d\t" `cat $QUAST_REPORT | head -22 | tail -1 | \
awk '{print $2}'` >> $STATS_OUT
# L50
printf "%d\t" `cat $QUAST_REPORT | head -26 | tail -1 | \
awk '{print $2}'` >> $STATS_OUT
# wuhCor1 match (%)
printf "%s\t" `cat $QUAST_REPORT | head -39 | tail -1 | \
awk '{print $4"%"}'` >> $STATS_OUT
# coverage x
printf "%s\n" $X >> $STATS_OUT
done
done
We can then perform some additional exploration of the results in R so as to inspect if the coverage increase really improves the results. Prior to creating some evaluation figures, we need to define two important measurements in genome assemblies:
-
L50: Given a set of contigs, each with its own length, the L50 count is defined as the smallest number of contigs whose length sum makes up half of genome size.
-
N50: N50 statistic defines assembly quality in terms of contiguity. Given a set of contigs, the N50 is defined as the sequence length of the shortest contig at 50% of the total genome length. It can be thought of as the point of half of the mass of the distribution; the number of bases from all contigs longer than the N50 will be close to the number of bases from all contigs shorter than the N50.
covidStats <- read.delim("files/stats.txt", check.names = FALSE)
# Boxplot of number of contigs
ggplot(data = covidStats) + geom_boxplot(aes(x = coverage, y = `number of contigs`,
color = coverage), outlier.size = 2) + geom_jitter(aes(x = coverage, y = `number of contigs`,
color = coverage), size = 0.5) + scale_y_continuous(breaks = seq(0, max(covidStats$`number of contigs`),
by = 2)) + ggtitle("Number of contigs per coverage")# Boxplot of L50 statistic
ggplot(data = covidStats) + geom_boxplot(aes(x = coverage, y = L50, color = coverage),
outlier.size = 2) + geom_jitter(aes(x = coverage, y = L50, color = coverage),
size = 0.5) + scale_y_continuous(breaks = seq(0, max(covidStats$L50), by = 2)) +
ggtitle("L50 per coverage")# Boxplot of N50 statistic
ggplot(data = covidStats) + geom_boxplot(aes(x = coverage, y = N50, color = coverage),
outlier.size = 2) + geom_jitter(aes(x = coverage, y = N50, color = coverage),
size = 0.5) + scale_y_continuous(trans = "log10") + ggtitle("N50 per coverage")# Boxplot of largest contig length
ggplot(data = covidStats) + geom_boxplot(aes(x = coverage, y = `largest contig length`,
color = coverage), outlier.size = 2) + geom_jitter(aes(x = coverage, y = `largest contig length`,
color = coverage), size = 0.5) + scale_y_continuous(trans = "log10") + ggtitle("Largest contig length")# Boxplot of match percentage with the reference genome
D <- covidStats
D$`wuhCor1 match (%)` <- as.numeric(gsub("%", "", D$`wuhCor1 match (%)`))
ggplot(data = D) + geom_boxplot(aes(x = coverage, y = `wuhCor1 match (%)`, color = coverage),
outlier.size = 2) + geom_jitter(aes(x = coverage, y = `wuhCor1 match (%)`, color = coverage),
size = 0.5) + ggtitle("Match with the reference genome")From the figures above we can extract the following results
- In all cases and with all metric inspected, 100x coverage gives the best outcomes. This is to be expected, given the length of the genome under investigation and how the assemblers work.
- The median number of contigs from the related assemblies population is 2. Also the majority of number of contigs for each case is concentrated in the range 1-4.
- The median L50 is 1, which means that the first contig captures the majority of the genome under investigation each time. The majoroty of L50 values is again mostly 1 and 2.
- The median N50 is close to 30kb in 100x and 200x coverage wiht the majority of N50s being concentrated very close to 30kb which is the length of the genome under investigation.
- The same observation as in (4) applies when we plot the largest contig length.
- If one inspects individual QUAST outputs and use (we leave this as exercise) BLAT to query the contigs of a couple of samples against the reference genome, we can see that the contigs are either complementary or slightly overlap but in most cases span the whole genome.
Therefore, we can state that the optimal coverage to use is 100x (with BBNorm normalization) and given the SARS-CoV-2 reference genome, it should be quite straightforward to complete the assemblies using tools such as Ragout.
In this section we will try to complete the de novo assemblies using the existing reference genome and MeDuSa and rerun QUAST to gather asembly statistics for later assessment. The following script template should do the work:
#!/bin/bash
HOME_PATH=/PATH/TO/ANALYSIS/DIRECTORY
ASSEMBLY_PATH=$HOME_PATH/assembly/100x
REFERENCE_PATH=$HOME_PATH/reference
GENOME_INDEX=$REFERENCE_PATH/wuhCor1.fa
MUMMER_PATH=/opt/ngstools/mummer
MEDUSA_PATH=/opt/ngstools/medusa
# Just to be sure
export PATH=$PATH:$MUMMER_PATH
for BASE in `ls $ASSEMBLY_PATH`
do
echo "Processing $BASE..."
SPADES_OUT=$ASSEMBLY_PATH/$BASE/spades/scaffolds.fasta
MEDUSA_OUT=$ASSEMBLY_PATH/$BASE/medusa
if [ -d $MEDUSA_OUT ]
then
rm -r $MEDUSA_OUT
fi
mkdir -p $MEDUSA_OUT
mkdir -p $MEDUSA_OUT/draft
# Could be a symbolic link if not working in SMB share
cp $GENOME_INDEX $MEDUSA_OUT/draft/
java -jar $MEDUSA_PATH/medusa.jar -v \
-f $MEDUSA_OUT/draft \
-i $SPADES_OUT \
-o $MEDUSA_OUT/medusa.fasta \
-scriptPath $MEDUSA_PATH/medusa_scripts
rm -r $MEDUSA_OUT/draft
# If medusa does not correct, it does not write anything to the output...
# In this case we must copy the original to the output to have a consistent
# output for QUAST
if [ ! -f $MEDUSA_OUT/medusa.fasta ]
then
cp $SPADES_OUT $MEDUSA_OUT/medusa.fasta
fi
$QUAST_COMMAND \
-r $GENOME_INDEX \
-o $MEDUSA_OUT/quast \
$MEDUSA_OUT/medusa.fasta
done
After MeDuSa attempt to complete the genomes, we are calculating again some statistics to assess the completion process.
#!/bin/bash
HOME_PATH=/PATH/TO/ANALYSIS/DIRECTORY
ASSEMBLY_PATH=$HOME_PATH/assembly/100x
MEDUSA_OUT="stats_100x_medusa.txt"
printf "%s\t%s\t%s\t%s\t%s\t%s\t%s\t%s\n" "name" "number of contigs" \
"largest contig length" "GC (%)" "N50" "L50" "wuhCor1 match (%)" > \
$MEDUSA_OUT
for BASE in `ls $ASSEMBLY_PATH`
do
echo "Processing $BASE..."
QUAST_100x=$ASSEMBLY_PATH/$BASE/medusa/quast/report.txt
# names
printf "%s\t" $BASE >> $MEDUSA_OUT
# # contigs
printf "%d\t" `cat $QUAST_100x | head -16 | tail -1 | \
awk '{print $3}'` >> $MEDUSA_OUT
# length of largest contig
printf "%d\t" `cat $QUAST_100x | head -17 | tail -1 | \
awk '{print $3}'` >> $MEDUSA_OUT
# GC content
printf "%s\t" `cat $QUAST_100x | head -20 | tail -1 | \
awk '{print $3"%"}'` >> $MEDUSA_OUT
# N50
printf "%d\t" `cat $QUAST_100x | head -22 | tail -1 | \
awk '{print $2}'` >> $MEDUSA_OUT
# L50
printf "%d\t" `cat $QUAST_100x | head -26 | tail -1 | \
awk '{print $2}'` >> $MEDUSA_OUT
# wuhCor1 match (%)
printf "%s\n" `cat $QUAST_100x | head -39 | tail -1 | \
awk '{print $4"%"}'` >> $MEDUSA_OUT
done
Then, we perform some comparisons.
covidRaw <- read.delim("files/stats.txt", check.names = FALSE)
covidMed <- read.delim("files/stats_100x_medusa.txt", check.names = FALSE)
covidStats <- rbind(covidRaw[, -ncol(covidRaw)], covidMed)
covidStats$source <- rep(c("SPAdes", "SPAdes+MeDuSa"), c(nrow(covidRaw), nrow(covidMed)))
# Boxplot of number of contigs
ggplot(data = covidStats) + geom_boxplot(aes(x = source, y = `number of contigs`,
color = source), outlier.size = 2) + geom_jitter(aes(x = source, y = `number of contigs`,
color = source), size = 0.5) + scale_y_continuous(breaks = seq(0, max(covidStats$`number of contigs`),
by = 2)) + ggtitle("Number of contigs per pipeline")# Boxplot of L50 statistic
ggplot(data = covidStats) + geom_boxplot(aes(x = source, y = L50, color = source),
outlier.size = 2) + geom_jitter(aes(x = source, y = L50, color = source), size = 0.5) +
scale_y_continuous(breaks = seq(0, max(covidStats$L50), by = 2)) + ggtitle("L50 per source")# Boxplot of N50 statistic
ggplot(data = covidStats) + geom_boxplot(aes(x = source, y = N50, color = source),
outlier.size = 2) + geom_jitter(aes(x = source, y = N50, color = source), size = 0.5) +
scale_y_continuous(trans = "log10") + ggtitle("N50 per source")# Boxplot of largest contig length
ggplot(data = covidStats) + geom_boxplot(aes(x = source, y = `largest contig length`,
color = source), outlier.size = 2) + geom_jitter(aes(x = source, y = `largest contig length`,
color = source), size = 0.5) + scale_y_continuous(trans = "log10") + ggtitle("Largest contig length")# Boxplot of match percentage with the reference genome
D <- covidStats
D$`wuhCor1 match (%)` <- as.numeric(gsub("%", "", D$`wuhCor1 match (%)`))
ggplot(data = D) + geom_boxplot(aes(x = source, y = `wuhCor1 match (%)`, color = source),
outlier.size = 2) + geom_jitter(aes(x = source, y = `wuhCor1 match (%)`, color = source),
size = 0.5) + ggtitle("Match with the reference genome")It is evident that almost all the assemblies have been improved with the help of the reference genome. However, from the last plot we can see that there are some samples which show poorer concordance with the reference genome. They can be easily identified:
D$name[which(D$`wuhCor1 match (%)` < 95)]
## [1] 19_S4 19_S4 AL11_S66 AL15_S68 19_S4 AL11_S66 AL1_S60 AL8_S63
## [9] 19_S4
## 85 Levels: 1_S1 10_S51 11_S36 13_S24 14_S25 16_S45 17_S3 19_S4 2_S29 ... L8_S73Sometimes, the genome completion process introduces N's. We can assess this with the following R code (this should run in the scripts folder of this tutorial and follow the notation introduced in the template scripts):
library(Biostrings)
homeDir <- "../assembly/100x/"
samples <- dir(homeDir)
alphaList <- vector("list", length(samples))
names(alphaList) <- samples
for (s in samples) {
tmp <- readDNAStringSet(file.path(homeDir, s, "medusa", "medusa.fasta"))
alphaList[[s]] <- alphabetFrequency(tmp[[1]])
}
alphabet <- do.call("rbind", alphaList)
table(alphabet[, "N"])
##
## 0 99 100 200 298 300 399 400 495 500 1300
## 50 1 14 5 1 6 1 2 1 3 1
rownames(alphabet)[which(alphabet[, "N"] > 100)]
## [1] "17_S3" "19_S4" "26_S39" "34_S50" "36_S8" "37_S43"
## [7] "39_S35" "67_S53" "68_S18" "75_S55" "76_S56" "79_S59"
## [13] "AL15_S68" "C8-20_S83" "C8-3_S78" "L14_S76" "L24_S77" "L25_S84"
## [19] "L31_S79" "L32_S80"Generally, the number and length of gaps in such small genomes such as the SARS-CoV-2 can be the results of several reasons. Some typical ones are:
- Non-uniform sequencing coverage across the viral genome
- Poor quality reads spanning the gap areas
- Problems in the capture kit design
As we have demonstrated, using the initial samples without normalization results
in poor quality assemblies. Another way of dealing with excessive gaps is to be
more lenient during the normalization procedure, by allowing reads of less
optimal depth to be part of normalized reads that are used for the assembly.
This can be controlled through the mindepth, minkmers and percentile
options of BBNorm. We are using these in the template script that will follow
for guided de novo genome assembly.
In the previous parts, we discussed how we can perform and basically assess
de novo genome assembly of SARS-CoV-2. We also discussed how we can refine the
process based on the existing reference genome. Now, we are going to make
further use of the reference genome by providing it to SPAdes with the option
--trusted-contigs. We are going to combine this procedure with the more
lenient normalization strategy discussed above. The following script template
should complete the work. We assume 100x coverage.
#!/bin/bash
HOME_PATH=/PATH/TO/ANALYSIS/DIRECTORY
REFERENCE_PATH=$HOME_PATH/reference
FASTQ_PATH=$HOME_PATH/fastq_qual
ASSEMBLY_PATH=$HOME_PATH/assembly_trusted
GENOME_INDEX=$REFERENCE_PATH/wuhCor1.fa
BBNORM_COMMAND=/opt/ngstools/bbmap/bbnorm.sh
SPADES_COMMAND=/opt/ngstools/spades/spades.py
QUAST_COMMAND=/opt/ngstools/quast/quast.py
CORES=16
if [ ! -d $ASSEMBLY_PATH ]
then
mkdir -p $ASSEMBLY_PATH
fi
for BASE in `ls $FASTQ_PATH`
do
echo "Processing $BASE"
F1=$FASTQ_PATH/$BASE/$BASE"_R1.fastq"
F2=$FASTQ_PATH/$BASE/$BASE"_R2.fastq"
pigz -d $F1".gz" $F2".gz"
for DP in 100
do
echo "Processing $DPx"
CURRENT_OUTPATH=$ASSEMBLY_PATH/$DP"x"/$BASE
mkdir -p $CURRENT_OUTPATH
$BBNORM_COMMAND \
in=$F1 \
in2=$F2 \
out=$CURRENT_OUTPATH/$BASE"_R1.fastq" \
out2=$CURRENT_OUTPATH/$BASE"_R2.fastq" \
min=5 \
target=$DP \
fixspikes=t \
deterministic=t \
threads=$CORES \
mindepth=1 \
minkmers=2 \
percentile=30
$SPADES_COMMAND \
-1 $CURRENT_OUTPATH/$BASE"_R1.fastq.gz" \
-2 $CURRENT_OUTPATH/$BASE"_R2.fastq.gz" \
-o $CURRENT_OUTPATH/spades \
--threads $CORES --careful \
--trusted-contigs $GENOME_INDEX
$QUAST_COMMAND \
-r $GENOME_INDEX \
-o $CURRENT_OUTPATH/quast \
$CURRENT_OUTPATH/spades/scaffolds.fasta
pigz $CURRENT_OUTPATH/$BASE"_R1.fastq" $CURRENT_OUTPATH/$BASE"_R2.fastq"
done
pigz $F1 $F2
done
Then, based on QUAST output we can again gather some assembly quality statistics:
#!/bin/bash
HOME_PATH=/PATH/TO/ANALYSIS/DIRECTORY
ASSEMBLY_PATH=$HOME_PATH/assembly_trusted
STATS_OUT="files/stats_trusted.txt"
printf "%s\t%s\t%s\t%s\t%s\t%s\t%s\t%s\n" "name" "number of contigs" \
"largest contig length" "GC (%)" "N50" "L50" "wuhCor1 match (%)" \
"coverage" > $STATS_OUT
for X in `ls $ASSEMBLY_PATH`
do
echo "Processing $X..."
for BASE in `ls $ASSEMBLY_PATH/$X`
do
echo " Processing $BASE..."
QUAST_REPORT=$ASSEMBLY_PATH/$X/$BASE/quast_greek/report.txt
# names
printf "%s\t" $BASE >> $STATS_OUT
# # contigs
printf "%d\t" `cat $QUAST_REPORT | head -16 | tail -1 | \
awk '{print $3}'` >> $STATS_OUT
# length of largest contig
printf "%d\t" `cat $QUAST_REPORT | head -17 | tail -1 | \
awk '{print $3}'` >> $STATS_OUT
# GC content
printf "%s\t" `cat $QUAST_REPORT | head -20 | tail -1 | \
awk '{print $3"%"}'` >> $STATS_OUT
# N50
printf "%d\t" `cat $QUAST_REPORT | head -22 | tail -1 | \
awk '{print $2}'` >> $STATS_OUT
# L50
printf "%d\t" `cat $QUAST_REPORT | head -26 | tail -1 | \
awk '{print $2}'` >> $STATS_OUT
# wuhCor1 match (%)
printf "%s\t" `cat $QUAST_REPORT | head -39 | tail -1 | \
awk '{print $4"%"}'` >> $STATS_OUT
# coverage x
printf "%s\n" $X >> $STATS_OUT
done
done
The following table presents the gathered statistics:
library(kableExtra)
tab <- read.delim("files/stats_trusted.txt",check.names=FALSE)
kable(tab) %>%
kable_styling(bootstrap_options=c("striped","hover","condensed")) %>%
row_spec(4,bold=TRUE,color="white",background="#00B400") %>%
row_spec(83,bold=TRUE,color="white",background="#B40000")
By inspecting the summary statistics we can see that in almost all cases, the
SARS-CoV-2 genome is captured by the aforementioned process and there is more
than 99.9% match in most cases. There is one particular case, the sample
L40_S81. Although the match with the reference is almost 100%, N50 is only
15621. This suggests that there are 2 overlapping contigs spanning the whole
viral genome and for some reason, the assembler was not able to merge them.
There is also the sample 14_S25 where we have two large contigs, but in this
case N50 is 29914 so the second contig should be an artifact.
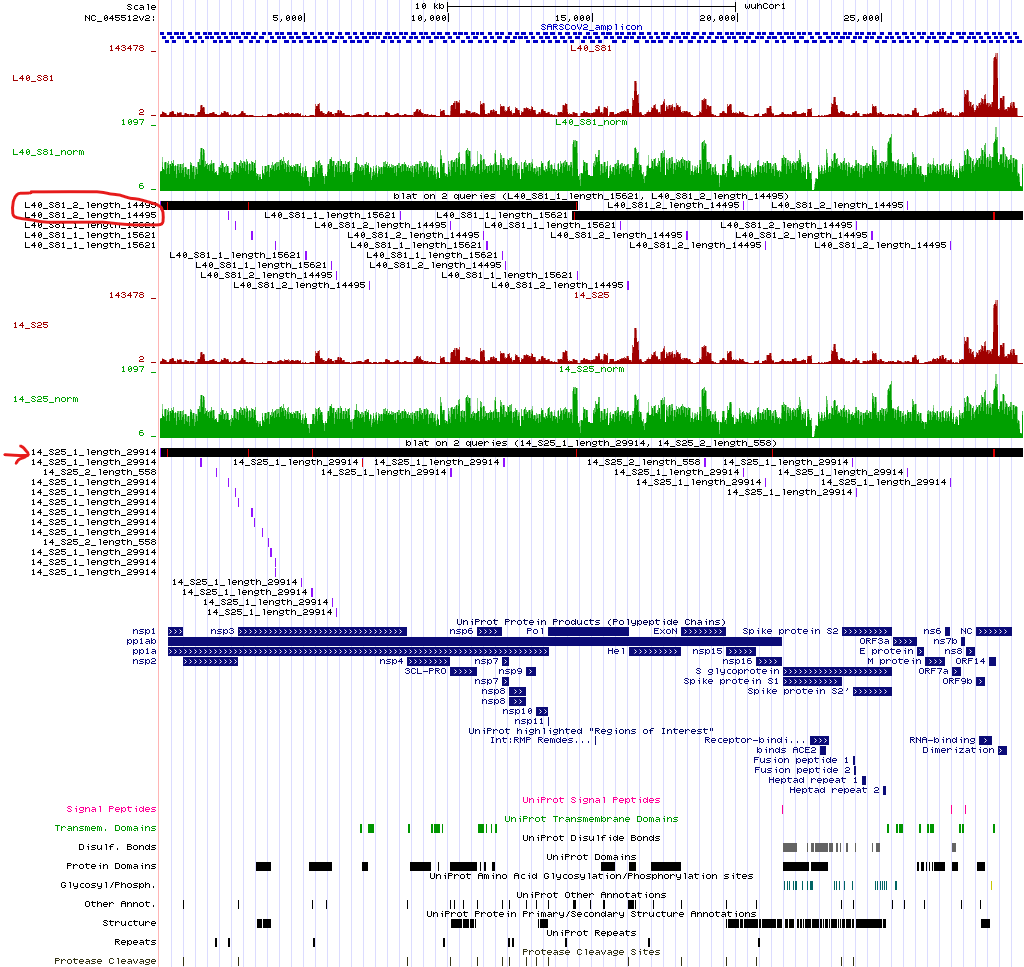
To verify our hypothesis about samples L40_S81 and 14_S25, we can align the
contigs in the reference genome using BLAT on the UCSC Genome Browser website.
We get the following results:
Indeed, L40_S81 comprises two large contigs. We are going to try to merge the
two contigs using MeDuSa:
HOME_PATH=/PATH/TO/ANALYSIS/DIRECTORY
REFERENCE_PATH=$HOME_PATH/reference
ASSEMBLY_PATH=$HOME_PATH/assembly_trusted
cd $ASSEMBLY_PATH/100x/L40_S81/spades
mkdir -p medusa_L40_S81/draft
cp $REFERENCE_PATH/wuhCor1.fa ./medusa_L40_S81/draft/
java -jar /PATH/TO/MEDUSA/medusa.jar \
-f ./medusa_L40_S81/draft \
-i ./scaffolds.fasta \
-o ./medusa_L40_S81/medusa.fasta \
-scriptPath /PATH/TO/MEDUSA/medusa_scripts/
Medusa will merge the two contigs but will add Ns. It will also produce a
significantly larger genome as it will also try to merge some artifacts in the
end of scaffolds file. This can be easily remedied manually by using BLAT
against the wuhCor1 reference genome and the trim the additional sequence
beyond the end of the polyA ending of the SARS-CoV-2 genome.
Finally, we gather the final FASTA files, ready for any downstream analysis. We
can use the Biostrings Bioconductor package to read, process and extract the
largest contigs representing the assembly. We should not forget to replace the
original scaffolds.fasta output of SPAdes with the one from MeDuSa (we backup
the original file first):
HOME_PATH=/PATH/TO/ANALYSIS/DIRECTORY
ASSEMBLY_PATH=$HOME_PATH/assembly_trusted
cd $ASSEMBLY_PATH/100x/L40_S81/spades
cp scaffolds.fasta scaffolds.fasta.bak
cp ./medusa_L40_S81/medusa.fasta scaffolds.fasta
and then within R:
if (!requireNamespace("BiocManager",quietly=TRUE))
install.packages("BiocManager")
BiocManager::install("Biostrings")
HOME_PATH <- "/PATH/TO/ANALYSIS/DIRECTORY"
ASSEMBLY_PATH <- file.path(HOME_PATH,"assembly_trusted","100x")
FASTA_PATH <- file.path(HOME_PATH,"final_fasta")
if (!dir.exists(FASTA_PATH))
dir.create(FASTA_PATH)
setwd(ASSEMBLY_PATH)
samples <- dir()
for (s in samples) {
message("Reading ",s)
tmp <- readDNAStringSet(file.path(s,"spades","scaffolds.fasta"))
tmp <- tmp[1]
names(tmp) <- s
message("Writing ",s)
writeXStringSet(tmp,filepath=file.path(FASTA_PATH,paste0(s,".fasta")))
}