QIIME 2 plugin for PCA analysis of protein sequences, as described by Wang & Kennedy (see below).
Install mamba into your base environment (if not already done):
conda install mamba -n base -c conda-forgeCreate a new conda environment and install q2-protein-pca with its dependencies:
mamba create -yn protein-pca \
-c conda-forge -c bioconda -c https://packages.qiime2.org/qiime2/2022.4/tested -c defaults \
q2-protein-pca q2cli q2-emperor
conda activate protein-pcaTo see actions available within the q2-protein-pca plugin:
qiime dev refresh-cache
qiime protein-pca --helpThis repository contains a sample dataset of thioredoxin sequences that can be used to try out the plugin's functionality.
The data is located under sample_data: thioredoxin-seqs.fasta contains 22 protein sequences and thioredoxin-metadata.tsv
contains corresponding metadata (to aid with the visualisation).
First, we import the data into a QIIME 2 artifact:
qiime tools import --input-path thioredoxin-seqs.fasta --output-path thioredoxin-seqs.qza --type "FeatureData[ProteinSequence]"
We can generate a multiple sequence alignment, using the mafft action as follows:
qiime protein-pca mafft --i-sequences thioredoxin-seqs.qza --o-alignment thioredoxin-aln.qza
This sequence alignment needs to be ranked to convert amino acids in letter representation to their respective ranks, as described by Wang & Kennedy:
qiime protein-pca rank-alignment --i-sequences thioredoxin-aln.qza --o-ranked-alignment thioredoxin-ranked.qza
Next, we perform principal component analysis on the rank matrix received by running the previous command. This should generate two types of outputs - PCA scores and PCA loadings (both of which can be visualised later):
qiime protein-pca pca --i-ranks thioredoxin-ranked.qza --o-pca-scores thioredoxin-pca-scores.qza --o-pca-loadings thioredoxin-pca-loadings.qza
Additionally, we generate position mapping between amino acid positions in the alignment and their respective positions within original protein sequences:
qiime protein-pca map-positions --i-aligned-sequences thioredoxin-aln.qza --o-mapped-positions thioredoxin-mapped.qza
To visualise PCA scores we can use plot action from the emperor plugin. This will generate a plot on which we can
visualise how different sequences group together. This is where we require the metadata file - in this example we will
be able to see how thioredoxins from different kingdoms/domains of life cluster together:
qiime emperor plot --i-pcoa thioredoxin-pca-scores.qza --m-metadata-file thioredoxin-metadata.tsv --o-visualization thioredoxin-pca-scores.qzv
The more interesting question, however, is which amino acids within thioredoxin's sequence contribute most and least to this variation. In other words, which thioredoxin residues make the protein sequence to be classified as bacterial vs. animal or which residues are most conserved within the sequence (e.g. could we identify active center amino acids?). To do that we can analyse PCA loadings by running the following command:
qiime protein-pca plot-loadings --i-pca-loadings thioredoxin-pca-loadings.qza --i-positions-mapping thioredoxin-mapped.qza --o-visualization thioredoxin-pca-loadings.qzv
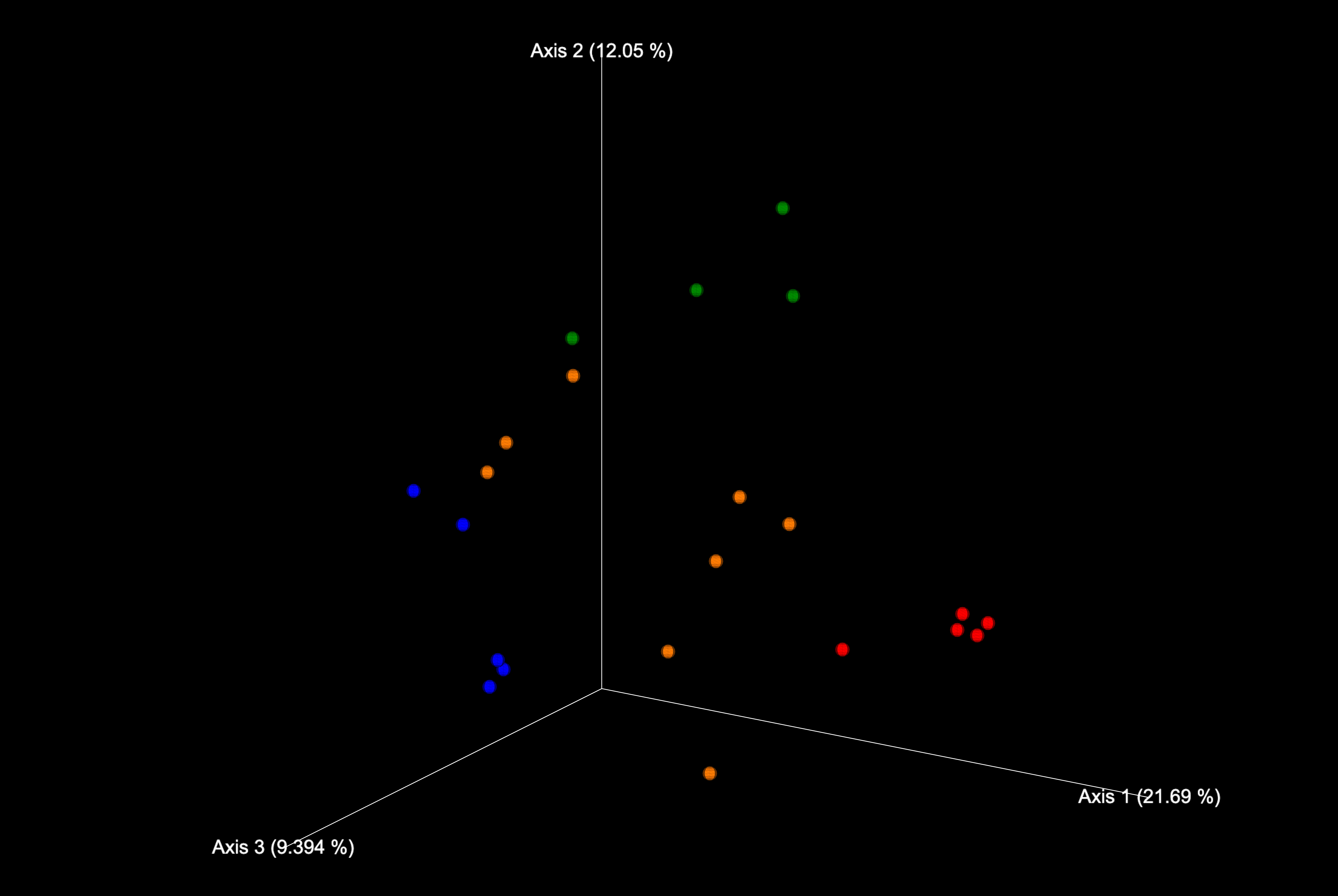
The scores plot obtained in the first visualisation should look something like:
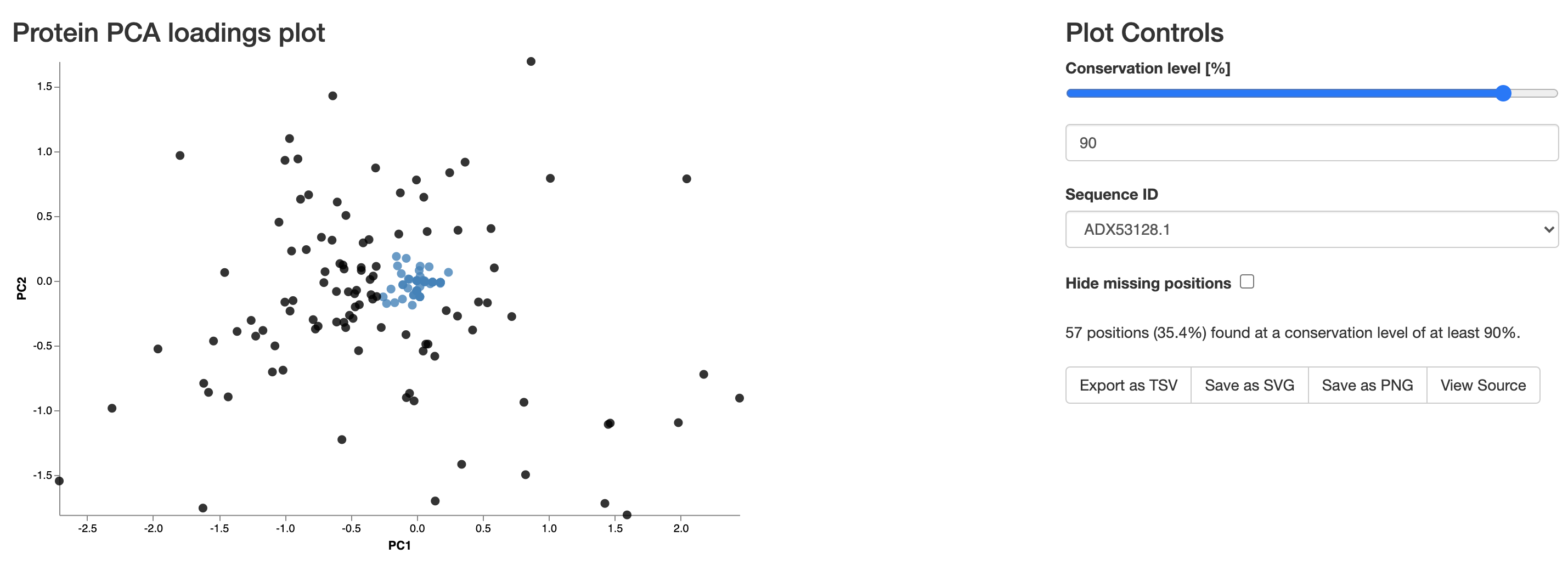
Different colours represent different kingdoms. Using the second plot we can identify the most conserved positions within the thioredoxin sequence:
On the visualisation page you can select the level of conservation you are interested in: setting to 90% would highlight all positions whose distance from the PC space origin does not exceed more than 10% of the distance to the furthest point. Additionally, selecting a protein id from the drop-down menu will show you in the table below which amino acid positions in that sequence were within that level (highlighted in red). In this example you can see how the two cysteines in thioredoxin's active centre are strictly conserved in all organisms (positions 33 and 36 in E. coli's thioredoxin, ADX53128.1). Also, the two prolines important for maintaining thioredoxin's redox properties and stability appear as (nearly)-strictly conserved (positions 41 and 77).
If there is an exisitng protein structure deposited in the Protein Data Bank that you would like to use to show the conserved positions, you can do so by specifying two additional parameters:
--p-pdb-id- a four letter code representing the ID of a structure file you want to fetch from PDB (e.g. 2trx)--p-nterm-offset- a number indicating how many amino acids are missing from the N-terminus in the PDB structure (the default is 1 as most often the initial methionine is not present; you should increase this number if a larger piece of the N-terminus was removed; if the methionine is present, set to 0)
Our command to generate this visualisation would now look like:
qiime protein-pca plot-loadings --i-pca-loadings thioredoxin-pca-loadings.qza --i-positions-mapping thioredoxin-mapped.qza --o-visualization thioredoxin-pca-loadings.qzv --p-pdb-id 2trx --p-nterm-offset 1
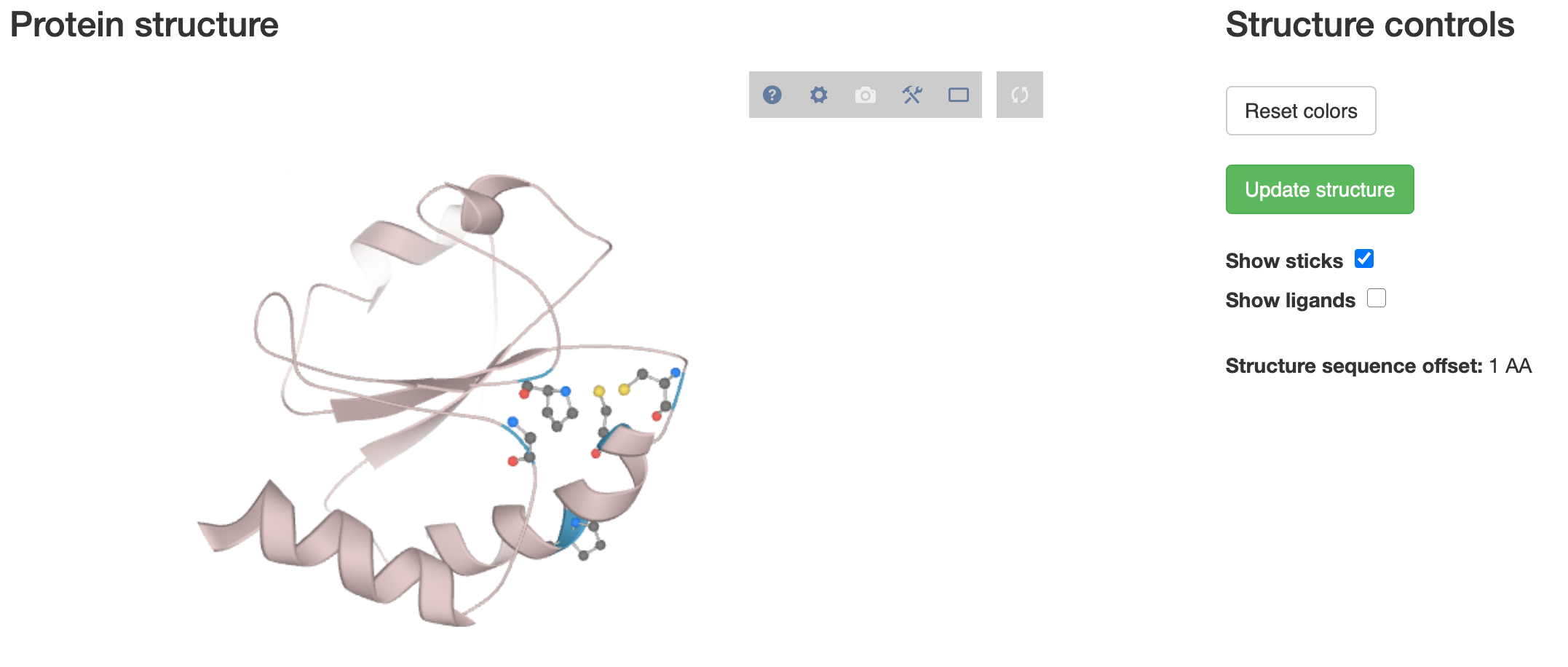
Once you open the visualisation you should see an additional panel containing your protein structure of choice:
Initially nothing will be overlayed - above that panel you should first select an accession number corresponding to the structure fetched. Clicking Update structure will re-render the 3D panel. You will need to update structure every time after adjusting the conservation threshold - that is to prevent constant re-rendering of the visualisation (could be slow for large and complex proteins).
Important: remember to select the correct accession id using the dropdown next to the PCA plot. Otherwise the numbering of residues highlighted in the structure may be wrong.
For more details about the ranking method that this plugin implements, head to Wang & Kennedy (2014). If you want to see another, more realistic example of how this method can be applied, check out Ziemski et al. (2018) which analysed an unknown, actinobacterial protein family in the context of a large AAA+ protein superfamily. If you want to read more about thioredoxins themselves, you can start by going through this review: Collet & Messens (2010).
- B. Wang & M.A. Kennedy (2014). Principal components analysis of protein sequence clusters. J Struct Funct Genomics, 15(1), 1-11. doi:10.1007/s10969-014-9173-2
- M. Ziemski, A. Jomaa, D. Mayer, S. Rutz, C. Giese, D. Veprintsev & E. Weber-Ban (2018). Cdc48-like protein of actinobacteria (Cpa) is a novel proteasome interactor in mycobacteria and related organisms. eLife 7, e34055. doi:10.7554/eLife.34055
- J.F. Collet & J. Messens (2010). Structure, Function, and Mechanism of Thioredoxin Proteins. Antioxidants & Redox Signaling 13(8). doi.org/10.1089/ars.2010.3114
- D. Sehnal, M. Deshpande, R.S. Vařeková, S. Mir, K. Berka, A. Midlik, L. Pravda, S. Velankar & J. Koča (2017). LiteMol suite: interactive web-based visualization of large-scale macromolecular structure data. Nat Methods 14, 1121-1122. doi:10.1038/nmeth.4499
- PDB LiteMol