This repo is deprecated.
If you need help finishing a project using Cenote-Taker 2, I will still field questions/troubleshoot (open an issue).
Otherwise:
Please use Cenote-Taker 3. It's great!!
######### ######### ######### ######### ######### ######### ######### ######### #########
######### ######### ######### ######### ######### ######### ######### ######### #########
######### ######### ######### ######### ######### ######### ######### ######### #########
######### ######### ######### ######### ######### ######### ######### ######### #########
######### ######### ######### ######### ######### ######### ######### ######### #########
######### ######### ######### ######### ######### ######### ######### ######### #########
######### ######### ######### ######### ######### ######### ######### ######### #########
######### ######### ######### ######### ######### ######### ######### ######### #########
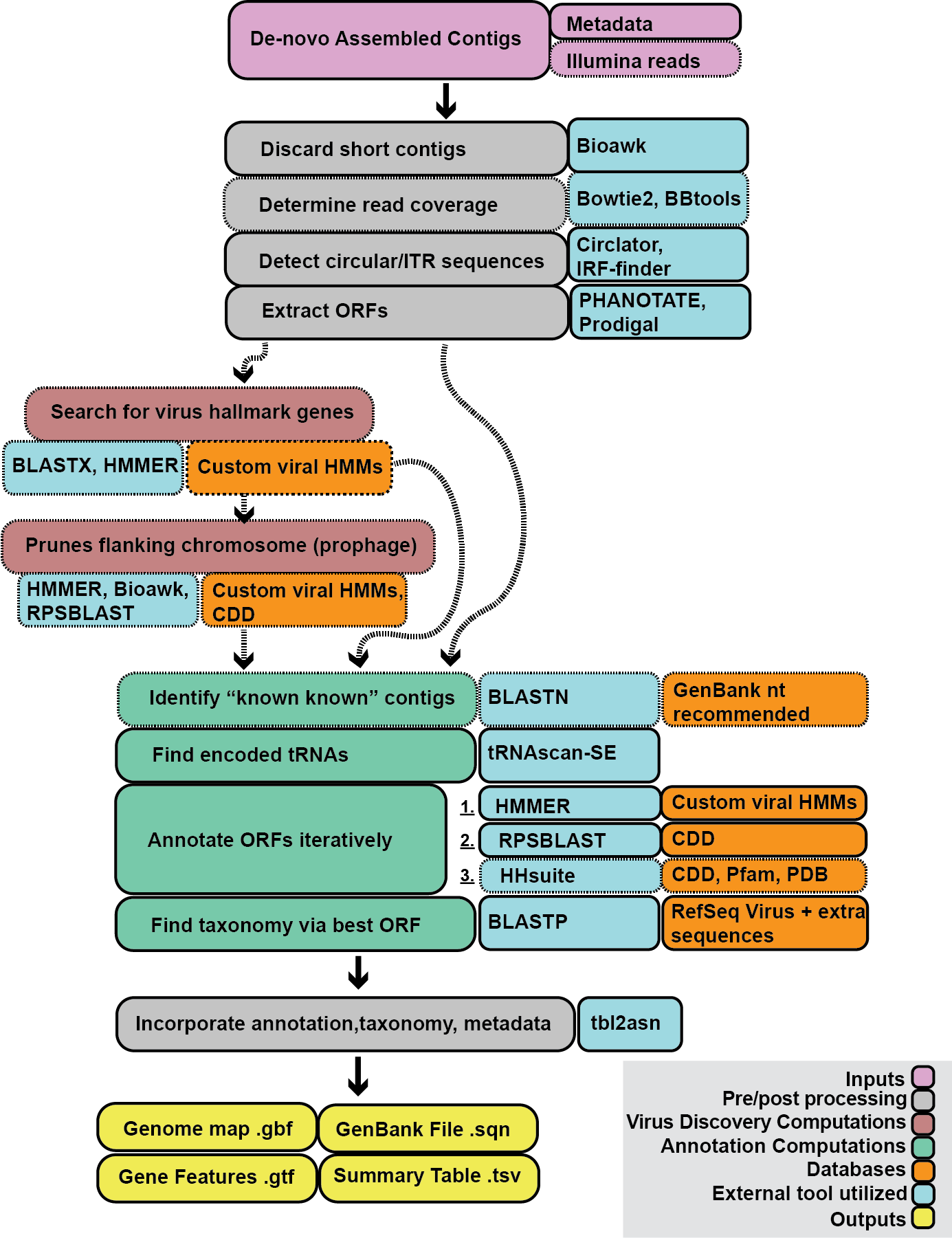
Cenote-Taker 2 is a dual function bioinformatics tool. On the one hand, Cenote-Taker 2 can discover/predict virus sequences from any kind of genome or metagenomic assembly. On the other hand, virus sequences/genomes (perhaps predicted by another tool?) can be annotated with a variety of sequences features, genes, and taxonomy. Either the discovery or the the annotation module can be used independently.
+ The code is currently functional. Feel free to use Cenote-Taker 2 at will.
+ Major update on May 6th 2022: Version 2.1.5
+ Cenote-Taker 2.1.5 has an easier, more reliable installation and database downloads.
+ Some packages that have given many users issues have been replaced. Taxonomy is more flexible. See release notes.
+ "virion" is now default databaseIf you just want to discover/predict virus sequences and get a report on those sequences, use Cenote Unlimited Breadsticks, also provided in the Cenote-Taker 2 repo.
If you just want to annotate your virus sequences and make genome maps, run Cenote-Taker 2 using -am True.
An ulterior motive for creating and distributing Cenote-Taker 2 is to facilitate annotation and deposition of viral genomes into GenBank where they can be used by the scientific public. Therefore, I hope you consider depositing the submittable outputs (.sqn) after reviewing them. I am not affiliated with GenBank.
See "Use Cases" below, and read the Cenote-Taker 2 wiki for useful information on using the pipeline (e.g. expected outputs) and screeds on myriad topics. Using a HPC with at least 16 CPUs and 16g of dedicated memory is recommended for most runs. (Annotation of a few selected genomes or virus discovery on smaller databases can be done with less memory/CPU in a reasonable amount of time).
To update from v2.1.3 (note that biopython and bedtools are now required):
conda activate cenote-taker2_env
pip install phanotate
conda install -c conda-forge -c bioconda hhsuite last=1282 seqkit
cd Cenote-Taker2
git pull
#Then update the BLAST database (see instructions below).
Update to HMM databases (hallmark genes) occurred on June 16th, 2021. Update to the BLAST (taxonomy) database occurred on May 6th, 2022. See instructions below to update your database.
Read the manuscript in Virus Evolution
If you cannot or do not want to install and run this on the command line, Cenote-Taker 2 v 2.1.3 is freely available to run with point-and-clink interface on the CyVerse Discovery Environment.
** Databases will require between 8GB (most basic) and 75GB (all the optional databases) of storage.
** Don't install without checking conda version first.
** Install on machine running on Linux (with a reasonably new OS). Support for MacOS is forthcoming.
If you just want a lightweight (7GB), faster, NON-ANNOTATING virus discovery tool, use Cenote Unlimited Breadsticks. The Unlimited Breadsticks module is included in the Cenote-Taker 2 repo, so no need to install it if you already have Cenote-Taker 2 (you may need to update from older versions Cenote-Taker2)
- ALERT *** If you choose to install all optional databases for HHsuite,
- installation will take about 2 hours due to slow download speeds for pdb70
- AND require about 75GB of storage space. -
Change to the directory you'd like to be the parent to the install directory
-
Ensure Conda is installed and working (required for installation and execution of
Cenote-Taker 2). Use version 4.10 or better. Note: instructions for installing Conda are probably specific to your university's/organization's requirements, so it is always best to ask your IT professional or HPC administrator. Generally, you will want to install Miniconda in your data directory.
conda -V
- Clone the
Cenote-Taker 2github repo.
git clone https://github.com/mtisza1/Cenote-Taker2.git
- Install the conda environment (phanotate, last, and hhsuite don't play nice with the .yml file, so they need special commands)
conda env create --file cenote-taker2_env.yml
# follow conda prompts to allow install
conda activate cenote-taker2_env
pip install phanotate
conda install -c conda-forge -c bioconda hhsuite last=1282
- Change to the
Cenote-Taker2repo directory OR a different location where you want the databases to be stored. (NOTE: if you install the databases in a custom location you will need to specify this directory each time you run the tool) Download the databases.
conda activate cenote-taker2_env
cd Cenote-Taker2
**choose one of the following**
# with all the options (75GB). The PDB database (--hhPDB) takes about 2 hours to download.
python update_ct2_databases.py --hmm True --protein True --rps True --taxdump True --hhCDD True --hhPFAM True --hhPDB True
# substantially smaller but with some hhsuite DBs (20GB). I recommend this if you are unsure which you want.
python update_ct2_databases.py --hmm True --protein True --rps True --taxdump True --hhCDD True --hhPFAM True
# only the required DBs, No hhsuite (8GB)
python update_ct2_databases.py --hmm True --protein True --rps True --taxdump True
- THIS HAS NOT BEEN UPDATED RECENTLY. BIOCONDA VERSION NOT RECOMMENDED AT THE MOMENT *
A user has packaged Cenote-Taker 2 in Bioconda for use by their institute. However, installation can be done by anyone using their package with a few commands. All the above alerts, requirements, and warnings still apply. This will also require a user to have 32GB of storage in their default conda environment directory.
Commands:
conda create -n cenote-taker2 -c hcc -c conda-forge -c bioconda -c defaults cenote-taker2=2020.04.01
conda activate cenote-taker2
download-db.sh
The Krona database directory will then need to be manually downloaded and set up. This should work:
CT2_DIR=$PWD
KRONA_DIR=$( which python | sed 's/bin\/python/opt\/krona/g' )
cd ${KRONA_DIR}
sh updateTaxonomy.sh
cd ${KRONA_DIR}
sh updateAccessions.sh
cd ${CT2_DIR}
Discussion: LINK
As of now, the HMM database has been updated from the original (update on June 16th, 2021), and the BLAST database has been updated (May 6th, 2022). This update should only take a minute or two. Here's how you update (modify if your conda environment is different than below example):
# update Cenote-Taker 2 (change to main repo directory):
git pull
# load your conda environment:
conda activate cenote-taker2_env
#change to Cenote-Taker2 directory
cd Cenote-Taker2
# run the update script:
python update_ct2_databases.py --hmm True --protein True
Cenote-Taker 2 currently runs in a python wrapper.
- Activate the Conda environment.
Check environments:
conda info --envs
#Default:
conda activate cenote-taker2_env
#Or if you've put your conda environment in a custom location:
conda activate /path/to/better/directory/cenote-taker2_env
- Run the python script to get the help menu (see options below).
# quick help menu
python /path/to/Cenote-Taker2/run_cenote-taker2.py
# full help menu
python /path/to/Cenote-Taker2/run_cenote-taker2.py -h
- Run some contigs. For example:
python /path/to/Cenote-Taker2/run_cenote-taker2.py -c MY_CONTIGS.fasta -r my_contigs1_ct -m 32 -t 32 -p true -db virion
#Or, if you want to save a log of the run, add "2>&1 | tee output.log" to the end of the command:
python /path/to/Cenote-Taker2/run_cenote-taker2.py -c MY_CONTIGS.fasta -r my_contigs1_ct -m 32 -t 32 -p true -db virion 2>&1 | tee output.log
If you just want to annotate your pre-selected virus sequences and make genome maps, run Cenote-Taker 2 using -am True.
Example:
# clip and wrap circular sequences
python /path/to/Cenote-Taker2/run_cenote-taker2.py -c MY_VIRUSES.fasta -r viruses_am_ct -m 32 -t 32 -p False -am True
# do not wrap circular sequences, but label DTR regions
python /path/to/Cenote-Taker2/run_cenote-taker2.py -c MY_VIRUSES.fasta -r viruses_am_ct -m 32 -t 32 -p False -am True --wrap False
For very divergent genomes, setting -hh hhsearch will marginally improve number of genes that are annotated. This setting increasese the run time quite a bit. On the other hand, setting -hh none will skip the time consuming hhblits step. With this, you'll still get pretty good genome maps, and might be most appropriate for very large virus genome databases, or for runs where you just want to do a quick check.
Virus-like particle (VLP) prep assembly:
-p False -db standard
You might apply a size cutoff for linear contigs as well, e.g. --minimum_length_linear 3000 OR --minimum_length_linear 5000. Changing length minima does not affect false positive rates, but short linear contigs may not be useful, depending on your goals.
Example:
python /path/to/Cenote-Taker2/run_cenote-taker2.py -c MY_VLP_ASSEMBLY.fasta -r my_VLP1_ct -m 32 -t 32 -p False -db standard --minimum_length_linear 3000
Whole genome shotgun (WGS) metagenomic assembly:
-p True -db virion --minimum_length_linear 3000 --lin_minimum_hallmark_genes 2
While you should definitely definitely prune virus sequences from WGS datasets, CheckV also does a very good job (I'm still formally comparing these approaches) and you could use --prune_prophage False on a metagenome assembly and feed the unpruned contigs from Unlimited Breadsticks into checkv end_to_end if you prefer. My suggestion is to prune with Cenote-Taker 2, then run CheckV.
Example with prune:
python /path/to/Cenote-Taker2/run_cenote-taker2.py -c MY_WGS_ASSEMBLY.fasta -r my_WGS1_ct -m 32 -t 32 -p True -db virion --minimum_length_linear 3000 --lin_minimum_hallmark_genes 2
Bacterial isolate genome or MAG
-p True -db virion --minimum_length_linear 3000 --lin_minimum_hallmark_genes 2
Using --lin_minimum_hallmark_genes 1 -db virion with WGS or bacterial genome data will (in my experience) yield very few sequences that appear to be false positives, however, there are lots of "degraded" prophage sequences in these sequencing sets, i.e. some/most genes of the phage have been lost. That said, sequence with just 1 hallmark gene is neither a guarantee of a degraded phage (especially in the case of ssDNA viruses) nor is 2+ hallmark a guarantee of of a complete phage.
Example:
python /path/to/Cenote-Taker2/run_cenote-taker2.py -c MY_BACTERIAL_GENOME.fasta -r my_genome1_ct -m 32 -t 32 -p True -db virion --minimum_length_linear 3000 --lin_minimum_hallmark_genes 2
RNAseq assembly of any kind (if you only want RNA viruses)
-p False -db rna_virus
If you also want DNA virus transcripts, or if your data is mixed RNA/DNA sequencing, you might do a run with -db rna_virus, then, from this run, take the file "other_contigs/non_viral_domains_contigs.fna" and use it as input for another run with -db virion.
Example:
python /path/to/Cenote-Taker2/run_cenote-taker2.py -c MY_METATRANSCRIPTOME.fasta -r my_metatrans1_ct -m 32 -t 32 -p False -db rna_virus
Vcontact2 is a popular downstream tool for clustering phage genomes into genus-level bins. Here's an example of how to prepare files from Cenote-Taker 2.
# change directory to a Cenote-Taker 2 output directory
# specify summary file (name based on run title):
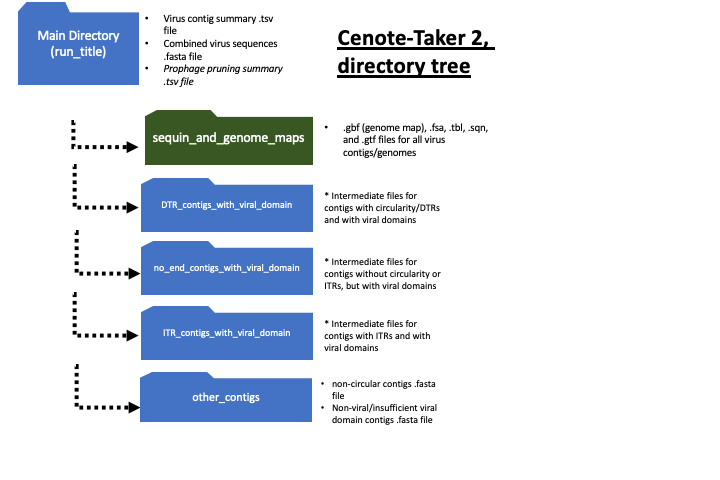
ls *_CONTIG_SUMMARY.tsv
SUMMARY="cenote_out_CONTIG_SUMMARY.tsv"
# make files for VContact2
if [ -s vcontact2_gene_to_genome1.csv ] || [ -s vcontact2_all_proteins.faa ] ; then echo "vcontact2 files already exist. NOT overwriting." ; else echo "protein_id,contig_id,keywords" > vcontact2_gene_to_genome1.csv ; tail -n+2 $SUMMARY | cut -f2,4 | while read VIRUS END ;do if [[ "$END" == "DTR" ]] ; then AA=$( find . -type f -name "${VIRUS}.rotate.AA.sorted.fasta" ) ; else AA=$( find . -type f -name "${VIRUS}.AA.sorted.fasta" ) ; fi ; grep -F ">" $AA | cut -d " " -f1 | sed 's/>//g' | while read LINE ; do echo "${LINE},${VIRUS}" ; done >> vcontact2_gene_to_genome1.csv ; cat $AA >> vcontact2_all_proteins.faa ; done ; fi
usage: run_cenote-taker2.py [-h]
-c ORIGINAL_CONTIGS
-r RUN_TITLE
-p PROPHAGE
-m MEM
-t CPU
[-am ANNOTATION_MODE]
[--template_file TEMPLATE_FILE]
[--reads1 F_READS]
[--reads2 R_READS]
[--minimum_length_circular CIRC_LENGTH_CUTOFF]
[--minimum_length_linear LINEAR_LENGTH_CUTOFF]
[-db VIRUS_DOMAIN_DB]
[--lin_minimum_hallmark_genes LIN_MINIMUM_DOMAINS]
[--circ_minimum_hallmark_genes CIRC_MINIMUM_DOMAINS]
[--known_strains HANDLE_KNOWNS]
[--blastn_db BLASTN_DB]
[--enforce_start_codon ENFORCE_START_CODON]
[-hh HHSUITE_TOOL]
[--crispr_file CRISPR_FILE]
[--isolation_source ISOLATION_SOURCE]
[--Environmental_sample ENVIRONMENTAL_SAMPLE]
[--collection_date COLLECTION_DATE]
[--metagenome_type METAGENOME_TYPE]
[--srr_number SRR_NUMBER]
[--srx_number SRX_NUMBER]
[--biosample BIOSAMPLE]
[--bioproject BIOPROJECT]
[--assembler ASSEMBLER]
[--molecule_type MOLECULE_TYPE]
[--data_source DATA_SOURCE]
[--filter_out_plasmids FILTER_PLASMIDS]
[--scratch_directory SCRATCH_DIR]
[--blastp BLASTP]
[--orf-within-orf ORF_WITHIN]
[--cenote-dbs CENOTE_DBS] [--wrap WRAP]
[--hallmark_taxonomy HALLMARK_TAX]
Cenote-Taker 2 is a pipeline for virus discovery and thorough annotation of viral contigs and genomes.
Visit https://github.com/mtisza1/Cenote-Taker2#use-case-suggestionssettings for suggestions about how to
run different data types and https://github.com/mtisza1/Cenote-Taker2/wiki to read more. Version 2.1.5
optional arguments:
-h, --help show this help message and exit
REQUIRED ARGUMENTS for Cenote-Taker2 :
-c ORIGINAL_CONTIGS, --contigs ORIGINAL_CONTIGS
Contig file with .fasta extension in fasta format - OR
- assembly graph with .fastg extension. Each header
must be unique before the first space character
-r RUN_TITLE, --run_title RUN_TITLE
Name of this run. A directory of this name will be
created. Must be unique from older runs or older run
will be renamed. Must be less than 18 characters,
using ONLY letters, numbers and underscores (_)
-p PROPHAGE, --prune_prophage PROPHAGE
True or False. Attempt to identify and remove flanking
chromosomal regions from non-circular contigs with
viral hallmarks (True is highly recommended for
sequenced material not enriched for viruses. Virus
enriched samples probably should be False (you might
check with ViromeQC). Also, please use False if
--lin_minimum_hallmark_genes is set to 0)
-m MEM, --mem MEM example: 56 -- Gigabytes of memory available for
Cenote-Taker2. Typically, 16 to 32 should be used.
Lower memory will work in theory, but could extend the
length of the run
-t CPU, --cpu CPU Example: 32 -- Number of CPUs available for Cenote-
Taker2. Approximately 32 CPUs should be used
moderately sized metagenomic assemblies. For large
datasets, increased performance can be seen up to 120
CPUs. Fewer than 16 CPUs will work in theory, but
could extend the length of the run. See GitHub repo
for suggestions.
OPTIONAL ARGUMENTS for Cenote-Taker2. Most of which are important to consider!!!
GenBank typically only accepts genome submission with ample metadata.
See https://www.ncbi.nlm.nih.gov/Sequin/sequin.hlp.html#ModifiersPage for more information on GenBank metadata fields:
-am ANNOTATION_MODE, --annotation_mode ANNOTATION_MODE
Default: False -- Annotate sequences only (skip
discovery). Only use if you believe each provided
sequence is viral
--template_file TEMPLATE_FILE
Template file with some metadata. Real one required
for GenBank submission. Takes a couple minutes to
generate: https://submit.ncbi.nlm.nih.gov/genbank/temp
late/submission/
--reads1 F_READS Default: no_reads -- ILLUMINA READS ONLY: First Read
file in paired read set - OR - read file in unpaired
read set - OR - read file of interleaved reads. Used
for coverage depth determination.
--reads2 R_READS Default: no_reads -- ILLUMINA READS ONLY: Second Read
file in paired read set. Disregard if not using paired
reads. Used for coverage depth determination.
--minimum_length_circular CIRC_LENGTH_CUTOFF
Default: 1000 -- Minimum length of contigs to be
checked for circularity. Bare minimun is 1000 nts
--minimum_length_linear LINEAR_LENGTH_CUTOFF
Default: 1000 -- Minimum length of non-circualr
contigs to be checked for viral hallmark genes.
-db VIRUS_DOMAIN_DB, --virus_domain_db VIRUS_DOMAIN_DB
default: virion -- 'standard' database: all virus (DNA
and RNA) hallmark genes (i.e. genes with known
function as virion structural, packaging, replication,
or maturation proteins specifically encoded by virus
genomes) with low false discovery rate. 'virion'
database: subset of 'standard', hallmark genes
encoding virion structural proteins, packaging
proteins, or capsid maturation proteins (DNA and RNA
genomes) with LOWEST false discovery rate. 'rna_virus'
database: For RNA virus hallmarks only. Includes RdRp
and capsid genes of RNA viruses. Low false discovery
rate.
--lin_minimum_hallmark_genes LIN_MINIMUM_DOMAINS
Default: 1 -- Number of detected viral hallmark genes
on a non-circular contig to be considered viral and
recieve full annotation. WARNING: Only choose '0' if
you have prefiltered the contig file to only contain
putative viral contigs (using another method such as
VirSorter or DeepVirFinder), or you are very confident
you have physically enriched for virus particles very
well (you might check with ViromeQC). Otherwise, the
duration of the run will be extended many many times
over, largely annotating non-viral contigs, which is
not what Cenote-Taker2 is meant for. For unenriched
samples, '2' might be more suitable, yielding a false
positive rate near 0.
--circ_minimum_hallmark_genes CIRC_MINIMUM_DOMAINS
Default:1 -- Number of detected viral hallmark genes
on a circular contig to be considered viral and
recieve full annotation. For samples physically
enriched for virus particles, '0' can be used, but
please treat circular contigs without known viral
domains cautiously. For unenriched samples, '1' might
be more suitable.
--known_strains HANDLE_KNOWNS
Default: do_not_check_knowns -- do not check if
putatively viral contigs are highly related to known
sequences (via MEGABLAST). 'blast_knowns': REQUIRES '
--blastn_db' option to function correctly.
--blastn_db BLASTN_DB
Default: none -- Set a database if using '--
known_strains' option. Specify BLAST-formatted
nucleotide datase. Probably, use only GenBank 'nt'
database downloaded from ftp://ftp.ncbi.nlm.nih.gov/
or another GenBank formatted .fasta file to make
databse
--enforce_start_codon ENFORCE_START_CODON
Default: False -- For final genome maps, require ORFs
to be initiated by a typical start codon? GenBank
submissions containing ORFs without start codons can
be rejected. However, if True, important but
incomplete genes could be culled from the final
output. This is relevant mainly to contigs of
incomplete genomes
-hh HHSUITE_TOOL, --hhsuite_tool HHSUITE_TOOL
default: hhblits -- hhblits will query PDB, pfam, and
CDD to annotate ORFs escaping identification via
upstream methods. 'hhsearch': hhsearch, a more
sensitive tool, will query PDB, pfam, and CDD to
annotate ORFs escaping identification via upstream
methods. (WARNING: hhsearch takes much, much longer
than hhblits and can extend the duration of the run
many times over. Do not use on large input contig
files). 'no_hhsuite_tool': forgoes annotation of ORFs
with hhsuite. Fastest way to complete a run.
--crispr_file CRISPR_FILE
Tab-separated file with CRISPR hits in the following
format: CONTIG_NAME HOST_NAME NUMBER_OF_MATCHES. You
could use this tool:
https://github.com/edzuf/CrisprOpenDB. Then reformat
for Cenote-Taker 2
--isolation_source ISOLATION_SOURCE
Default: unknown -- Describes the local geographical
source of the organism from which the sequence was
derived
--Environmental_sample ENVIRONMENTAL_SAMPLE
Default: False -- True or False, Identifies sequence
derived by direct molecular isolation from an
unidentified organism
--collection_date COLLECTION_DATE
Default: unknown -- Date of collection. this format:
01-Jan-2019, i.e. DD-Mmm-YYYY
--metagenome_type METAGENOME_TYPE
Default: unknown -- a.k.a. metagenome_source
--srr_number SRR_NUMBER
Default: unknown -- For read data on SRA, run number,
usually beginning with 'SRR' or 'ERR'
--srx_number SRX_NUMBER
Default: unknown -- For read data on SRA, experiment
number, usually beginning with 'SRX' or 'ERX'
--biosample BIOSAMPLE
Default: unknown -- For read data on SRA, sample
number, usually beginning with 'SAMN' or 'SAMEA' or
'SRS'
--bioproject BIOPROJECT
Default: unknown -- For read data on SRA, project
number, usually beginning with 'PRJNA' or 'PRJEB'
--assembler ASSEMBLER
Default: unknown_assembler -- Assembler used to
generate contigs, if applicable. Specify version of
assembler software, if possible.
--molecule_type MOLECULE_TYPE
Default: DNA -- viable options are DNA - OR - RNA
--data_source DATA_SOURCE
default: original -- original data is not taken from
other researchers' public or private database.
'tpa_assembly': data is taken from other researchers'
public or private database. Please be sure to specify
SRA metadata.
--filter_out_plasmids FILTER_PLASMIDS
Default: True -- True - OR - False. If True, hallmark
genes of plasmids will not count toward the minimum
hallmark gene parameters. If False, hallmark genes of
plasmids will count. Plasmid hallmark gene set is not
necessarily comprehensive at this time.
--scratch_directory SCRATCH_DIR
Default: none -- When running many instances of
Cenote-Taker2, it seems to run more quickly if you
copy the hhsuite databases to a scratch space
temporarily. Use this argument to set a scratch
directory that the databases will be copied to (at
least 100GB of scratch space are required for copying
the databases)
--blastp BLASTP Do not use this argument as of now.
--orf-within-orf ORF_WITHIN
Default: False -- Remove called ORFs without HMMSCAN
or RPS-BLAST hits that begin and end within other
ORFs? True or False
--cenote-dbs CENOTE_DBS
Default: cenote_script_path -- If you downloaded and
setup the databases in a non-standard location,
specify path
--wrap WRAP Default: True -- Wrap/rotate DTR/circular contigs so
the start codon of an ORF is the first nucleotide in
the contig/genome
--hallmark_taxonomy HALLMARK_TAX
Default: False -- Get hierarchical taxonomy
information for all hallmark genes? This report
(*.hallmarks.taxonomy.out) is not considered in the
final taxonomy call.
Michael J Tisza, Anna K Belford, Guillermo Domínguez-Huerta, Benjamin Bolduc, Christopher B Buck, Cenote-Taker 2 democratizes virus discovery and sequence annotation, Virus Evolution, Volume 7, Issue 1, January 2021, veaa100, https://doi.org/10.1093/ve/veaa100